Abstract
Polyphyllin I (PPI), an effective active ingredient in Paris polyphylla, has a diverse set of pharmacological properties. However, due to its poor solubility and oral absorption, its application and development are limited. In the study, we were committed to improving the solubility of PPI by developing a self-microemulsifying drug delivery system of PPI (PPI-SMEDDS), screening the best preparation process, and evaluating the quality and the in vivo pharmacokinetics of PPI, and PPI-SMEDDS following oral administration to rats were also studied. In addition, the pharmacological activities against human lung adenocarcinoma cell A549 in vitro were assessed. The best formulation had 15.89% ethyl oleate, 47.38% Cremophor RH40, and 36.73% 1,2 propylene glycol. The produced PPI-SMEDDS was clear and transparent, with an average particle size of 24.51 nm and a zeta potential of −17.54 ± 0.51 mV. In vitro, the cumulative release rate of PPI-SMEDDS was nearly 80% within 2 h. PPI-SMEDDS had a substantially greater area under the curve than PPI following oral treatment in rats, and the relative bioavailability of PPI in rats was 278.99%. More importantly, the anti-tumor effect of PPI-SMEDDS in vitro was significantly greater than that of PPI. These findings suggested that PPI-SMEDDS has the potential to improve the solubility, oral bioavailability of PPI, and anti-tumor effect, laying the groundwork for future research on the new PPI dosage form.
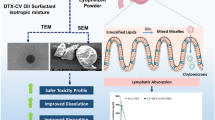
Graphical abstract













Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Gao XY, Zhang X, Chen W, Li J, Yang WJ, Zhang XW, Li SY, Liu CN. Transcriptome analysis of Paris polyphylla var. yunnanensis illuminates the biosynthesis and accumulation of steroidal saponins in rhizomes and leaves. Phytochemistry. 2020;178:112460.
Luo H, Xu Y, Sun DY, Cheng Y, Sun ZL, Gao J, Zhang YJ, Wang X. Assessment of the inhibition risk of paris saponins, bioactive compounds from Paris polyphylla, on CYP and UGT enzymes via cocktail inhibition assays. Regul Toxicol Pharmacol. 2020;113:104637.
Wang Q, Zhou X, Zhao YJ, Xiao J, Lu Y, Shi Q, Wang YJ, Wang HY, Liang QQ. Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-κB pathway. Front Immunol. 2018;9:2091.
Yang SY, Jiang Y, Yu XQ, Zhu LP, Wang L, Mao JZ, Wang MM, Zhou NH, Yang ZL, Liu Y, Zhu TT. Polyphyllin I inhibits propionibacterium acnes-induced IL-8 secretion in HaCaT cells by downregulating the CD36/NOX1/ROS/NLRP3/IL-1β pathway. Evid Based Complement Alternat Med. 2021;2021:1821220.
Lai L, Shen QP, Wang YJ, Chen LT, Lai JJ, Wu ZB, Jiang H. Polyphyllin I reverses the resistance of osimertinib in non-small cell lung cancer cell through regulation of PI3K/Akt signaling. Toxicol Appl Pharmacol. 2021;419:115518.
Wang WP, Liu Y, You LT, Sun MY, Qu CH, Dong XX, Yin XB, Ni J. Inhibitory effects of Paris saponin I, II, VI and VII on HUVEC cells through regulation of VEGFR2, PI3K/AKT/mTOR, Src/eNOS, PLCγ/ERK/MERK, and JAK2-STAT3 pathways. Biomed Pharmacother. 2020;131:110750.
Li JL, Ma WL, Cheng XM, Zhang XB, Xie Y, Ji ZG, Wu S. Activation of FOXO3 pathway is involved in polyphyllin I-induced apoptosis and cell cycle arrest in human bladder cancer cells. Arch Biochem Biophys. 2020;687:108363.
Wu YZ, Si Y, Xiang YC, Zhou T, Liu XW, Wu MW, et al. Polyphyllin I activates AMPK to suppress the growth of non-small-cell lung cancer via induction of autophagy. Arch Biochem Biophys. 2020;687:108285.
Mundada VP, Patel MH, Mundada PK, Sawant KK. Development of self-microemulsifying drug delivery system to improve nisoldipine bioavailability: cell line and in vivo evaluations: development of self-microemulsifying drug delivery system. AAPS PharmSciTech. 2021;22:256.
Yi T, Zhang J. Effects of hydrophilic carriers on structural transitions and in vitro properties of solid self-microemulsifying drug delivery systems. Pharm. 2019;11:267.
Wang LZ, Yan WR, Tian YR, Xue HH, Tang JH, Zhang LW. Self-microemulsifying drug delivery system of phillygenin: formulation development, characterization and pharmacokinetic evaluation. Pharm. 2020;12:130.
Chou YC, Li S, Ho CT, Pan MH. Preparation and evaluation of self-microemulsifying delivery system containing 5-demethyltangeretin on inhibiting xenograft tumor growth in mice. Int J Pharm. 2020;579:119134.
Liu J, Wang QL, Omari-Siaw E, Adu-Frimpong M, Liu J, Xu XM, et al. Enhanced oral bioavailability of Bisdemethoxycurcumin-loaded self-microemulsifying drug delivery system: formulation design, in vitro and in vivo evaluation. Int J Pharm. 2020;590:119887.
Wang M, You SK, Lee HK, Han MG, Lee HM, Pham T, et al. Development and evaluation of docetaxel-phospholipid complex loaded self-microemulsifying drug delivery system: optimization and in vitro/ex vivo studies. Pharm. 2020;12:544.
Zhu ZG, Liu J, Yang YH, Adu-Frimpong M, Ji H, Toreniyazov E, et al. SMEDDS for improved oral bioavailability and anti-hyperuricemic activity of licochalcone A. J Microencapsul. 2021;38:459–71.
Ye J, Gao Y, Ji M, Yang YF, Wang ZH, Wang BL, et al. Oral SMEDDS promotes lymphatic transport and mesenteric lymph nodes target of chlorogenic acid for effective T-cell antitumor immunity. J Immunother Cancer. 2021;9:e002753.
Rosso A, Almouazen E, Pontes J, Andretto V, Leroux M, Romasko E, et al. Supersaturable self-microemulsifying delivery systems: an approach to enhance oral bioavailability of benzimidazole anticancer drugs. Drug Deliv Transl Res. 2021;11:675–91.
Kamal MM, Nazzal S. Novel sulforaphane-enabled self-microemulsifying delivery systems (SFN-SMEDDS) of taxanes: formulation development and in vitro cytotoxicity against breast cancer cells. Int J Pharm. 2018;536:187–98.
Sawatdee S, Atipairin A, Sae Yoon A, Srichana T, Changsan N, Suwandecha T. Formulation development of albendazole-loaded self-microemulsifying chewable tablets to enhance dissolution and bioavailability. Pharm. 2019;11:134.
Zhu Y, Xu W, Zhang J, Liao Y, Firempong CK, Adu-Frimpong M, et al. Self-microemulsifying drug delivery system for improved oral delivery of limonene: preparation, characterization, in vitro and in vivo evaluation. AAPS PharmSciTech. 2019;20:153.
Yang Z, Wang Y, Cheng J, Shan B, Wang Y, Wang R, et al. Solid self-microemulsifying drug delivery system of Sophoraflavanone G: prescription optimization and pharmacokinetic evaluation. Eur J Pharm Sci. 2019;136:104953.
Park SY, Jin CH, Goo YT, Chae BR, Yoon HY, Kim CH, et al. Supersaturable self-microemulsifying drug delivery system enhances dissolution and bioavailability of telmisartan. Pharm Dev Technol. 2021;26:60–8.
Yu FL, Gong WL, Xu FJ, Wu JW, Shakya S, Zhu H. Influence of nutritional status on the absorption of Polyphyllin I, an anticancer candidate from Paris polyphylla in rats. Eur J Drug Metab Pharmacokinet. 2018;43:587–97.
Uzor S, Porazinski SR, Li L, Clark B, Ajiro M, Iida K, et al. CDC2-like (CLK) protein kinase inhibition as a novel targeted therapeutic strategy in prostate cancer. Sci Rep. 2021;11:7963.
Dan W, Shi L, Wang L, Wu D, Huang X, Zhong Y. PP7080 expedites the proliferation and migration of lung adenocarcinoma cells via sponging miR-670-3p and regulating UHRF1BP1. J Gene Med. 2021;23:e3341.
Zhang J, Hou L, Liang R, Chen X, Zhang R, Chen W, et al. CircDLST promotes the tumorigenesis and metastasis of gastric cancer by sponging miR-502-5p and activating the NRAS/MEK1/ERK1/2 signaling. Mol Cancer. 2019;18:80.
Qu L, Zhang W, Li J, Liu P. The miR-146b-5p promotes Ewing’s sarcoma cells progression via suppressing the expression of BTG2. Sci Prog. 2021;104:368504211002043.
Kalepu S, Manthina M, Padavala V. Oral lipid based drug delivery systems: an overview. Acta Pharm Sin B. 2013;3(6):361–72.
Li L, Wu J, Zheng F, Tang Q, Wu W, Hann SS. Inhibition of EZH2 via activation of SAPK/JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J Exp Clin Cancer Res. 2016;35(1):112.
Lai L, Shen Q, Wang Y, Chen L, Lai J, Wu Z, et al. Polyphyllin I reverses the resistance of osimertinib in non-small cell lung cancer cell through regulation of PI3K/Akt signaling. Toxicol Appl Pharmacol. 2021;419:115518.
Li HS, Xu Y. Inhibition of EZH2 via the STAT3/HOTAIR signalling axis contributes to cell cycle arrest and apoptosis induced by polyphyllin I in human non-small cell lung cancer cells. Steroids. 2020;164:108729.
Feng FF, Cheng P, Sun C, Wang H, Wang W. Inhibitory effects of polyphyllins I and VII on human cisplatin-resistant NSCLC via p53 upregulation and CIP2A/AKT/mTOR signaling axis inhibition. Chin J Nat Med. 2019;17(10):768–77.
Lei CQ, Wu X, Zhong X, Jiang L, Zhong B, Shu HB. USP19 inhibits TNF-α and IL-1β triggered NF-κB activation by deubiquitinating TAK1. J Immunol. 2019;203:259–68.
Huang L, Cai Y, Luo Y, Xiong D, Hou Z, Lv J, et al. JAZF1 suppresses papillary thyroid carcinoma cell proliferation and facilitates apoptosis via regulating TAK1/NF-κB pathways. Onco Targets Ther. 2019;12:10501–14.
Xu YR, Lei CQ. TAK1-TABs complex: a central signalosome in inflammatory responses. Front Immunol. 2021;11:608976.
Yang YP, Liu YB, Yu HH, Xie QL, Wang B, Jiang S, et al. Sesquiterpenes from Kadsura coccinea attenuate rheumatoid arthritis-related inflammation by inhibiting the NF-κB and JAK2/STAT3 signal pathways. Phytochemistry. 2021;194:113018.
Liu D, Shi K, Fu M, Chen F. Melatonin indirectly decreases gastric cancer cell proliferation and invasion via effects on cancer-associated fibroblasts. Life Sci. 2021;277:119497.
Acknowledgements
The authors are sincerely thankful to the Animal Experiment Center, Affiliated Hospital of Shandong University of Traditional Chinese Medicine for providing the facility for carrying out the research work.
Funding
This study was supported by the National Natural Science Foundation of China (81873330, 82104506, and 82074066).
Author information
Authors and Affiliations
Contributions
Conceptualization, Xin Wang and Ping Sun; Methodology, Rui Zhang and Shu Wang; Data curation, Minju Gu and Yuan Li; Software, Xiuping Zhuang and Chao Chen; Writing-original draft, Xin Wang and Rui Zhang; Writing- review & editing, Peimin Yang and Gongling Guo. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal protocols were approved by the Animal Center, Beijing SPF Weitonglihua Laboratory Animal Technology Co., Ltd. (Beijing, Certificate No. SCXK 2016–0006). All institutional and national guidelines for the care and use of laboratory animals were followed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Zhang, R., Wang, S. et al. Development, characterisation, and in vitro anti-tumor effect of self-microemulsifying drug delivery system containing polyphyllin I. Drug Deliv. and Transl. Res. 13, 356–370 (2023). https://doi.org/10.1007/s13346-022-01212-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01212-8