Abstract
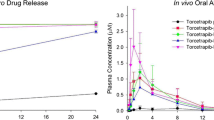
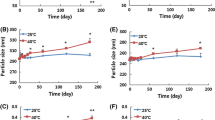
Ketamine is used as an analgesic adjuvant in patients with chronic cancer–related pain. However, ketamine’s short half-life requires frequent dose administration. Our aim was to develop a sustained release formulation of ketamine with high loading and to evaluate the in vivo pharmacokinetics and biodistribution in mice. Here, ketamine hydrochloride sustained-release lipid particles (KSL) were developed using the thin-film hydration method. The mean (± SD) encapsulation efficiency (EE) and drug loading (DL) of KSL were 65.6 (± 1.7)% and 72.4 (± 0.5)% respectively, and the mean (± SD) size of the lipid particles and the polydispersity index were 738 (± 137) nm and 0.44 (± 0.02) respectively. The release period of KSL in pH 7.4 medium was 100% complete within 8 h in vitro but a sustained-release profile was observed for more than 5 days after intravenous injection in mice. Importantly, the KSL formulation resulted in a 27-fold increase in terminal half-life, a threefold increase in systemic exposure (AUC0-∞), and a threefold decrease in clearance compared with the corresponding pharmacokinetics for intravenous ketamine itself. Our findings demonstrate high encapsulation efficiency of ketamine in the sustained-release KSL formulation with prolonged release in mice after systemic dose administration despite 100% in vitro release within 8 h that requires future investigation.
Graphical abstract






Similar content being viewed by others
Availability of data and materials
All the data are included in the main text or in the Supplementary Materials.
References
Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:381–91.
Jesorka A, Orwar O. Liposomes: technologies and analytical applications. Annu Rev Anal Chem (Palo Alto Calif). 2008;1:801–832.
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;1:65(1):36-48.
Collier M, Bachelder EM, Ainslie KM. Electrosprayed myocet-like liposomes: an alternative to traditional liposome production. Pharm Res. 2017;34:419–426.
Yang CY, Wong CS, Chang JY, Ho ST. Intrathecal ketamine reduces morphine requirements in patients with terminal cancer pain. Can J Anaesth. 1996;43:379–83.
Thannikary L, Naik B. Complications in Anesthesia, 2nd. ed., Elsevier Inc., 2007.
Bell RF, Kalso EA. Ketamine for pain management. Pain Rep. 2018;3:e674–e674.
Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA, Gould TD. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–60.
Han FY, Thurecht KJ, Lam AL, Whittaker AK, Smith MT. Novel polymeric bioerodable microparticles for prolonged-release intrathecal delivery of analgesic agents for relief of intractable cancer-related pain. J Pharm Sci. 2015;104:2334–44.
Li J, Wang X, Zhang T, Wang C, Huang Z, Luo X, Deng Y. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10:81–98.
Maherani B, Arab-Tehrany E, Mozafari MR, Gaiani C, Linder M. Liposomes: a review of manufacturing techniques and targeting strategies. Current Nanosci. 2011;7:436–52.
Çağdaş M, Sezer AD, Bucak S. Application of nanotechnology in drug delivery. Intech Open Limited, London, 2014.
Mozafari M. Liposomes: an overview of manufacturing techniques. Cell Mol Biol Lett. 2005;10:711–9.
Han FY, Whittaker A, Howdle SM, Naylor A, Shabir-Ahmed A, Smith MT. Sustained-release hydromorphone microparticles produced by supercritical fluid polymer encapsulation. J Pharm Sci. 2018.
Fatima MT, Islam Z, Ahmad E, Barreto GE, Ashraf GM. Ionic gradient liposomes: Recent advances in the stable entrapment and prolonged released of local anesthetics and anticancer drugs. Biomedicine & Pharmacotherapy. 2018;107:34-43.
Ketamine [Internet]. DrugBank Online. 2005 [cited 2021 Nov 13]. Available from: https://go.drugbank.com/drugs/DB01221.
Barceloux D. Medical toxicology of drug abuse: synthesized chemicals and psychoactive plants, in: R. Palmer (Ed.). 2012;110–120.
Ketamine hydrochloride CAS#: 1867-66-9 [Internet]. Chemical Book. 2017 [cited 2021 Nov 13]. Available from: https://www.chemicalbook.com/ProductChemicalPropertiesCB4141970_EN.htm.
Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238-IN227.
Balakrishnan P, Shanmugam S, Lee WS, Lee WM, Kim JO, Oh DH, Kim D-D, Kim JS, Yoo BK, Choi H-G, Woo JS, Yong CS. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm. 2009;377:1–8.
Agarwal R, Katare OP, Vyas SP. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 2001;228:43–52.
Sorkin R, Kampf N, Klein J. Effect of cholesterol on the stability and lubrication efficiency of phosphatidylcholine surface layers. Langmuir. 2017;33:7459–67.
Leonenko ZV, Finot E, Ma H, Dahms TES, Cramb DT. Investigation of Temperature-Induced Phase Transitions in DOPC and DPPC Phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys J. 2004;86:3783–93.
Scarff CA, Fuller MJG, Thompson RF, Iadanza MG. Variations on negative stain electron microscopy methods: tools for tackling challenging systems. J Vis Exp. 2018;132:e57199.
Han FY, Liu Y, Kumar V, Xu W, Yang G, Zhao CX, Woodruff TM, Whittaker AK, Smith MT. Sustained-release ketamine-loaded nanoparticles fabricated by sequential nanoprecipitation. Int J Pharm. 2020;581:119291.
Kumar V, Lee JD, Clark RJ, Noakes PG, Taylor SM, Woodruff TM. Preclinical pharmacokinetics of complement C5a receptor antagonists PMX53 and PMX205 in mice. ACS Omega. 2020;5:2345–54.
Briuglia M-L, Rotella C, McFarlane A, Lamprou DA. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 2015;5:231–42.
Redondo-Morata L, Giannotti MI, Sanz F. Influence of cholesterol on the phase transition of lipid bilayers: a temperature-controlled force spectroscopy study. Langmuir. 2012;28:12851–60.
Chong PL, Choate D. Calorimetric studies of the effects of cholesterol on the phase transition of C(18):C(10) phosphatidylcholine. Biophys J. 1989;55:551–6.
Malm AV, Corbett JCW. Improved dynamic light scattering using an adaptive and statistically driven time resolved treatment of correlation data. Sci Rep-Uk. 2019;9:13519.
Raval N, Maheshwari R, Kalyane D, Youngren-Ortiz SR, Chougule MB, Tekade RK. Chapter 10 - Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In: Tekade RK, editor. Basic fundamentals of drug delivery. Academic Press; 2019;369–400.
Bilal MH, Alaneed R, Steiner J, Mäder K, Pietzsch M, Kressler J. Chapter Three - Multiple grafting to enzymatically synthesized polyesters. In: Bruns N, Loos K, editors. Methods in Enzymology. Academic Press; 2019;57–97.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari MR. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57.
Zhu M, Whittaker AK, Smith MT, Han FY. Bioerodable ketamine-loaded microparticles fabricated using dissolvable hydrogel template technology. J Pharm Sci. 2019;108(3):1220-6.
Han FY, Whittaker AK, Howdle SM, Naylor A, Shabir-Ahmed A, Zhang C, Smith MT. Formulation of bioerodible ketamine microparticles as an analgesic adjuvant treatment produced by supercritical fluid polymer encapsulation. Pharmaceutics. 2018:10.
Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154:123–40.
Mayne C, Arcario MJ, Mahinthichaichan P, Baylon JL, Vermaas JV, Navidpour L, Wen P-C, Thangapandian S, Tajkhorshid E. The cellular membrane as a mediator for small molecule interaction withmembrane proteins. Biochem Biophys Acta. 2016;1858:2290–304.
Suarez-Sharp S, Li M, Duan J, Shah H, Seo P. Regulatory experience with in vivo in vitro correlations (IVIVC) in new drug applications. AAPS J. 2016;18:1379–90.
Senior J. Fate and behavior of liposomes in vivo: a review of controlling factors. Crit Rev Ther Drug Carrier Syst. 1987;3:123–93.
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286.
Szebeni J, Moghimi SM. Liposome triggering of innate immune responses: a perspective on benefits and adverse reactions: biological recognition and interactions of liposomes. J Liposome Res. 2009;19:85–90.
Gabizon A, Tzemach D, Mak L, Bronstein M, Horowitz AT. Dose dependency of pharmacokinetics and therapeutic efficacy of pegylated liposomal doxorubicin (DOXIL) in murine models. J Drug Target. 2002;10:539–48.
Lu Y, Kim S, Park K. In vitro–in vivo correlation: perspectives on model development. Int J Pharm. 2011;418:142–8.
Shabbits JA, Chiu GNC, Mayer LD. Development of an in vitro drug release assay that accurately predicts in vivo drug retention for liposome-based delivery systems. J Control Release. 2002;84:161–70.
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:2–33.
Lister J. Amphotericin B Lipid Complex ( AbelceP) in the treatment of invasive mycoses: the North American experience. Eur J Haematol. 1996;56S:18–23.
Acknowledgements
The authors acknowledge the Queensland Government Smart State Research Facilities Programme for supporting CIPDD research infrastructure. CIPDD is also supported by Therapeutic Innovation Australia (TIA). TIA is supported by the Australian Government through the National Collaborative Research Infrastructure Strategy (NCRIS) program. A special acknowledgement to Mohammad Mobasseri for his insight into the preparation of liposomes as a starting point for this research. We also thank The University of Queensland for the financial support to complete this research.
Funding
This work was supported by a collaborative seeding grant to FYH, TK, and JF from The University of Queensland, Australia. FYH and WX were supported financially by a National Health and Medical Research Council (NHMRC) Grant [APP1107723], Australia. This work also received financial support from the Australian Research Council (LE0775684, LE110100028, LE110100033, LE140100087, LE160100168). T.M.W also received support from the National Health and Medical Research Council (APP1118881).
Author information
Authors and Affiliations
Contributions
FYH initiated and with JRF conceived the project. FYH, JRF, and FM designed and developed the formulation and FM performed the formulation. WX and FM performed in vitro characterisation with the support from AKW. FYH and VK designed in vivo studies. VK, WX, and CSC performed pharmacokinetic (PK) studies and analysis with support from TMW. FYH, JRF, and WX wrote the manuscript with input from MTS, and all authors edited the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animal experiments were performed in accordance with approval from the University of Queensland Animal Ethics Committee, Australia (the approval number is 241/18, and date of approval is 21 Aug 2018).
Consent for publication
All authors have agreed to publish this work.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, W., Maqbool, F., Kumar, V. et al. Sustained-release ketamine-loaded lipid-particulate system: in vivo assessment in mice. Drug Deliv. and Transl. Res. 12, 2518–2526 (2022). https://doi.org/10.1007/s13346-021-01093-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01093-3