Abstract
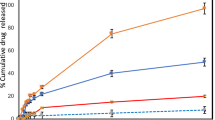
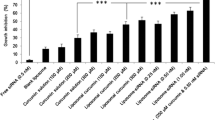
MicroRNAs (miRNAs) are involved in the pathogenesis of several cancers including skin squamous cell carcinoma (sSCC), and miR-134 is reported to possess tumor inhibition properties. The present study is an attempt to study the mechanistic role and antitumor property of miR-134 in sSCC. For this purpose, α-tocopherol PEG 1000 succinate (TPGS)-based PEGylated liposome was formulated and encapsulated with miR-134 (TP-miR-LP). CCK-8 assay results showed that miR-134 exhibited a concentration-dependent decrease in the cell viability of A-431 cells. Importantly, TPGS-based TP-miR-LP showed significantly (p < 0.05) lower cell viability compared with that of miR-134-loaded PEGylated liposome (miR-LP). Western blot analysis clearly indicates the specific targeting ability of miR-134 (TP-miR-LP) towards the Forkhead Box M1 (FOXM1) in the cancer cells. The apoptosis rate of the cells was significantly increased in TP-miR-LP (~ 38%) than that of miR-LP (~ 15%), respectively with significant inhibition of cell migration. Importantly, tumors treated with TP-miR-LP grew significantly slower compared with that of any other formulation group in the xenograft animal model. Present results clearly demonstrate the tumor suppressive effect of miR-134 through the downregulation of FOXM1 which subsequently blocks the downstream signaling pathways. These findings suggest the translational potential of miR-134 towards designing formulation strategies for sSCC treatment.

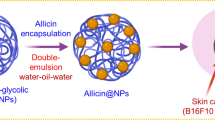
Graphical abstract







Similar content being viewed by others
Abbreviations
- miRNA:
-
microRNA
- sSCC:
-
skin squamous cell carcinoma
- TPGS:
-
α-tocopherol PEG 1000 succinate
- miR-LP:
-
miR-134-loaded PEGylated liposome
- TP-miR-LP:
-
miR-134-loaded TPGS-based PEGylated liposome
- Mut-LP:
-
mutant miR-134-loaded PEGylated liposome
- N/P ratio:
-
nitrogen/phosphate ratio
- MDR:
-
multidrug drug resistance
- FOXM1:
-
Forkhead Box M1
- EPR:
-
enhanced permeation and retention effect
References
Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–7.
Erb P, Ji J, Kump E, Mielgo A, Wernli M. Apoptosis and pathogenesis of melanoma and nonmelanoma skin cancer. Adv Exp Med Biol. 2008;624:283–295. https://doi.org/10.1007/978-0-387-77574-6_22.
Safwat MA, Soliman GM, Sayed D, Attia MA. Fluorouracil-loaded gold nanoparticles for the treatment of skin cancer: development, in vitro characterization, and in vivo evaluation in a mouse skin cancer xenograft model. Mol Pharm. 2018;15(6):2194–205.
Nasr S, Rady M, Gomaa I, Syrovets T, Simmet T, Fayad W, et al. Ethosomes and lipid-coated chitosan nanocarriers for skin delivery of a chlorophyll derivative: a potential treatment of squamous cell carcinoma by photodynamic therapy. Int J Pharm. 2019;568:118528.
Ci C, Wu C, Lyu D, Chang X, He C, Liu W, et al. Down-regulation of kynureninase restrains cutaneous squamous cell carcinoma proliferation and represses PI3K/AKT pathway. Clin Exp Dermatol. 2019;45:194–201. https://doi.org/10.1111/ced.14072.
Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38(7–8):802–32.
Ramasamy T, Ruttala HB, Kaliraj K, Poudel K, Jin SG, Choi HG, et al. Polypeptide derivative of metformin with the combined advantage of a gene carrier and anticancer activity. ACS Biomater Sci Eng. 2019;5:5159–68.
Gao Y, Liu T, Huang Y. MicroRNA-134 suppresses endometrial cancer stem cells by targeting POGLUT1 and notch pathway proteins. FEBS Lett. 2015;589:207–14.
Qin W, Ren Q, Liu T, Huang Y, Wang J. MicroRNA-155 is a novel suppressor of ovarian cancer-initiating cells that targets CLDN1. FEBS Lett. 2013;587:1434–9.
Zhang X, Zhang Y, Yang J, Li S, Chen J. Upregulation of miR-582-5p inhibits cell proliferation, cell cycle progression and invasion by targeting Rab27a in human colorectal carcinoma. Cancer Gene Ther. 2015;22:475–80.
Sanders DA, Ross-Innes CS, Beraldi D, Carroll JS, Balasubramanian S. Genome-wide mapping of FOXM1 binding reveals co-binding with estrogen receptor alpha in breast cancer cells. Genome Biol. 2013;14:R6.
Zhu D, Tao W, Zhang H, Liu G, Wang T, Zhang L, et al. Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomater. 2016;30:144–54.
Gormally MV, Dexheimer TS, Marsico G, Sanders DA, Lowe C, Matak-Vinkovic D, et al. Suppression of the FOXM1 transcriptional programme via novel small molecule inhibition. Nat Commun. 2014;5:5165.
Ahmed M, Hussain AR, Siraj AK, Uddin S, AlSanea N, Al-Dayel F, et al. Co-targeting of Cyclooxygenase-2 and FoxM1 is a viable strategy in inducing anticancer effects in colorectal cancer cells. Mol Cancer. 2015;14:131.
Zhang N, Xie Y, Li B, Ning Z, Wang A, Cui X. FoxM1 influences mouse hepatocellular carcinoma metastasis in vitro. Int J Clin Exp Pathol. 2015;8:2771–8.
Li J, Wang Y, Luo J, Fu Z, Ying J, Yu Y, et al. miR-134 inhibits epithelial to mesenchymal transition by targeting FOXM1 in non-small cell lung cancer cells. FEBS Lett. 2012;586(20):3761–5.
Chang C, Liu T, Huang Y, Qin W, Yang H, Chen J. MicroRNA-134-3p is a novel potential inhibitor of human ovarian cancer stem cells by targeting RAB27A. Gene. 2017;605:99–107.
Ramasamy T, Ruttala HB, Gupta B, Poudel BK, Choi HG, Yong CS, et al. Smart chemistry-based nanosized drug delivery systems for systemic applications: a comprehensive review. J Control Release. 2017;258:226–53.
Song XL, Ju RJ, Xiao Y, Wang X, Liu S, Fu M, et al. Application of multifunctional targeting epirubicin liposomes in the treatment of non-small-cell lung cancer. Int J Nanomedicine. 2017;12:7433–51.
Sayes CM, Aquino GV, Hickey AJ. Nanomaterial drug products: manufacturing and analytical perspectives. AAPS J. 2017;19(1):18–25.
Sayes CM, Staats H, Hickey AJ. Scale of health: indices of safety and efficacy in the evolving environment of large biological datasets. Pharm Res. 2014;31(9):2256–65.
Naseri Z, Oskuee R, Jaafari M, Moghadam M. Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int J Nanomedicine. 2018;13:7727–47.
Yang G, Yin B. Therapeutic effects of long-circulating miR-135a-containing cationic immunoliposomes against gallbladder carcinoma. Sci Rep. 2017;7(1):5982.
Ruttala HB, Chitrapriya N, Kaliraj K, Ramasamy T, Shin WH, Jeong JH, et al. Facile construction of bioreducible crosslinked polypeptide micelles for enhanced cancer combination therapy. Acta Biomater. 2017;63:135–49.
Al-Attar T, Madihally SV. Targeted cancer treatment using a combination of siRNA-liposomes and resveratrol-electrospun fibers in co-cultures. Int J Pharm. 2019;569:118599.
Assanhou AG, Li W, Zhang L, Xue L, Kong L, Sun H, et al. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials. 2015;73:284–95.
Gill KK, Kaddoumi A, Nazzal S. Mixed micelles of PEG(2000)-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: enhanced chemosenstization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. Eur J Pharm Sci. 2012;46(1–2):64–71.
Li Y, Tan X, Liu X, Liu L, Fang Y, Rao R, et al. Enhanced anticancer effect of doxorubicin by TPGS-coated liposomes with Bcl-2 siRNA-corona for dual suppression of drug resistance. Asian J Pharm Sci. 2019. https://doi.org/10.1016/j.ajps.2019.10.003.
Tan X, Fang Y, Ren Y, Li Y, Wu P, Yang X, et al. D-α-tocopherol polyethylene glycol 1000 succinate-modified liposomes with an siRNA corona confer enhanced cellular uptake and targeted delivery of doxorubicin via tumor priming. Int J Nanomedicine. 2019;14:1255–68.
Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer--a brief overview. Adv Biol Regul. 2015;57:1–9.
Ohtsuka M, Ling H, Doki Y, Mori M, Calin GA. MicroRNA processing and human cancer. J Clin Med. 2015;4(8):1651–67.
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. Forkhead box M1 transcription factor: a novel target for cancer therapy. Cancer Treat Rev. 2010;36:151–6.
Zhang Z, Zhang G, Kong C. FOXM1 participates in PLK1-regulated cell cycle progression in renal cell cancer cells. Oncol Lett. 2016;11:2685–91.
Funding
This study was supported by the Key Technologies R & D Program from the Department of Science and Technology of Henan Province (No: 162102310176).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the animal protocols for the experiments were approved by the Institutional Committee on Animal Research in the Henan University People’s Hospital, Zhengzhou.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jing, C., Yan, L., Wei, Z. et al. Exogenous delivery of microRNA-134 (miR-134) using α-tocopherol-based PEGylated liposome for effective treatment in skin squamous cell carcinoma. Drug Deliv. and Transl. Res. 11, 1000–1008 (2021). https://doi.org/10.1007/s13346-020-00811-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00811-7