Abstract
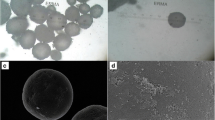
PD98059 is a reversible MEK inhibitor that we are investigating as a potential treatment for neurochemical changes in the brain that drive neurohumoral excitation in heart failure. In a rat model that closely resembles human heart failure, we found that central administration of PD98059 inhibits phosphorylation of ERK1/2 in the paraventricular nucleus of the hypothalamus, ultimately reducing sympathetic excitation which is a major contributor to clinical deterioration. Studies revealed that the pharmacokinetics and biodistribution of PD98059 match a two-compartment model, with drug found in brain as well as other body tissues, but with a short elimination half-life in plasma (approximately 73 min) that would severely limit its potential clinical usefulness in heart failure. To increase its availability to tissues, we prepared a sustained release PD98059-loaded PLGA microparticle formulation, using an emulsion solvent evaporation technique. The average particle size, yield percent, and encapsulation percent were found to be 16.73 μm, 76.6%, and 43%, respectively. In vitro drug release occurred over 4 weeks, with no noticeable burst release. Following subcutaneous injection of the microparticles in rats, steady plasma levels of PD98059 were detected by HPLC for up to 2 weeks. Furthermore, plasma and brain levels of PD98059 in rats with heart failure were detectable by LC/MS, despite expected erratic absorption. These findings suggest that PD98059-loaded microparticles hold promise as a novel therapeutic intervention countering sympathetic excitation in heart failure, and perhaps in other disease processes, including cancers, in which activated MAPK signaling is a significant contributing factor.

Graphical abstract




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. https://doi.org/10.1038/35065000.
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22(2):153–83. https://doi.org/10.1210/edrv.22.2.0428.
Cseh B, Doma E, Baccarini M. "RAF" neighborhood: protein-protein interaction in the Raf/Mek/Erk pathway. FEBS Lett. 2014;588(15):2398–406. https://doi.org/10.1016/j.febslet.2014.06.025.
Wu PK, Park JI. MEK1/2 inhibitors: molecular activity and resistance mechanisms. Semin Oncol. 2015;42(6):849–62. https://doi.org/10.1053/j.seminoncol.2015.09.023.
Blaukat A, Barac A, Cross MJ, Offermanns S, Dikic I. G protein-coupled receptor-mediated mitogen-activated protein kinase activation through cooperation of Galpha(q) and Galpha(i) signals. Mol Cell Biol. 2000;20(18):6837–48. https://doi.org/10.1128/mcb.20.18.6837-6848.2000.
Neuzillet C, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E. MEK in cancer and cancer therapy. Pharmacol Ther. 2014;141(2):160–71. https://doi.org/10.1016/j.pharmthera.2013.10.001.
Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17(7):1263–93. https://doi.org/10.1038/sj.leu.2402945.
Li L, Zhao GD, Shi Z, Qi LL, Zhou LY, Fu ZX. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol Lett. 2016;12(5):3045–50. https://doi.org/10.3892/ol.2016.5110.
Costigan DC, Dong F. The extended spectrum of RAS-MAPK pathway mutations in colorectal cancer. Genes, chromosomes & cancer. 2019. https://doi.org/10.1002/gcc.22813.
Li S, Balmain A, Counter CM. A model for RAS mutation patterns in cancers: finding the sweet spot. Nat Rev Cancer. 2018;18(12):767–77. https://doi.org/10.1038/s41568-018-0076-6.
Savoia P, Fava P, Casoni F, Cremona O. Targeting the ERK signaling pathway in melanoma. Int J Mol Sci. 2019;20(6). https://doi.org/10.3390/ijms20061483.
Wang AX, Qi XY. Targeting RAS/RAF/MEK/ERK signaling in metastatic melanoma. IUBMB Life. 2013;65(9):748–58. https://doi.org/10.1002/iub.1193.
Basu S, Harfouche R, Soni S, Chimote G, Mashelkar RA, Sengupta S. Nanoparticle-mediated targeting of MAPK signaling predisposes tumor to chemotherapy. Proc Natl Acad Sci U S A. 2009;106(19):7957–61. https://doi.org/10.1073/pnas.0902857106.
Cheng Y, Tian H. Current development status of MEK inhibitors. Molecules. 2017;22(10). https://doi.org/10.3390/molecules22101551.
Miao L, Tian H. Development of ERK1/2 inhibitors as a therapeutic strategy for tumour with MAPK upstream target mutations. J Drug Target. 2019;28:1–12. https://doi.org/10.1080/1061186X.2019.1648477.
Broman KK, Dossett LA, Sun J, Eroglu Z, Zager JS. Update on BRAF and MEK inhibition for treatment of melanoma in metastatic, unresectable, and adjuvant settings. Expert Opin Drug Saf. 2019;18(5):381–92. https://doi.org/10.1080/14740338.2019.1607289.
Li S, Liu S, Deng J, Akbay EA, Hai J, Ambrogio C, et al. Assessing therapeutic efficacy of MEK inhibition in a KRAS(G12C)-driven mouse model of lung cancer. Clin Cancer Res. 2018;24(19):4854–64. https://doi.org/10.1158/1078-0432.CCR-17-3438.
Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011;17(5):989–1000. https://doi.org/10.1158/1078-0432.CCR-10-2200.
Wei SG, Yu Y, Weiss RM, Felder RB. Inhibition of brain mitogen-activated protein kinase signaling reduces central endoplasmic reticulum stress and inflammation and sympathetic nerve activity in heart failure rats. Hypertension. 2016;67(1):229–36. https://doi.org/10.1161/HYPERTENSIONAHA.115.06329.
Wei SG, Yu Y, Weiss RM, Felder RB. Endoplasmic reticulum stress increases brain MAPK signaling, inflammation and renin-angiotensin system activity and sympathetic nerve activity in heart failure. Am J Physiol Heart Circ Physiol. 2016;311(4):H871–H80. https://doi.org/10.1152/ajpheart.00362.2016.
Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol. 2009;296(5):H1425–33. https://doi.org/10.1152/ajpheart.00942.2008.
Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension. 2008;52(4):679–86. https://doi.org/10.1161/HYPERTENSIONAHA.108.113639.
Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Angiotensin II-triggered p44/42 mitogen-activated protein kinase mediates sympathetic excitation in heart failure rats. Hypertension. 2008;52(2):342–50. https://doi.org/10.1161/HYPERTENSIONAHA.108.110445.
Yu Y, Wei SG, Zhang ZH, Weiss RM, Felder RB. ERK1/2 MAPK signaling in hypothalamic paraventricular nucleus contributes to sympathetic excitation in rats with heart failure after myocardial infarction. Am J Physiol Heart Circ Physiol. 2016;310(6):H732–9. https://doi.org/10.1152/ajpheart.00703.2015.
Yu Y, Xue BJ, Zhang ZH, Wei SG, Beltz TG, Guo F, et al. Early interference with p44/42 mitogen-activated protein kinase signaling in hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension. Hypertension. 2013;61(4):842–9. https://doi.org/10.1161/HYPERTENSIONAHA.111.00080.
Zhang ZH, Yu Y, Wei SG, Felder RB. Aldosterone-induced brain MAPK signaling and sympathetic excitation are angiotensin II type-1 receptor dependent. Am J Physiol Heart Circ Physiol. 2012;302(3):H742–51. https://doi.org/10.1152/ajpheart.00856.2011.
Felder RB, Yu Y, Zhang ZH, Wei SG. Pharmacological treatment for heart failure: a view from the brain. Clin Pharmacol Ther. 2009;86(2):216–20. https://doi.org/10.1038/clpt.2009.117.
Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295(1):H227–36. https://doi.org/10.1152/ajpheart.01157.2007.
Wei SG, Zhang ZH, Yu Y, Weiss RM, Felder RB. Central actions of the chemokine stromal cell-derived factor 1 contribute to neurohumoral excitation in heart failure rats. Hypertension. 2012;59(5):991–8. https://doi.org/10.1161/HYPERTENSIONAHA.111.188086.
Khaled KA, Sarhan HA, Ibrahim MA, Ali AH, Naguib YW. Prednisolone-loaded PLGA Microspheres. In vitro characterization and in vivo application in adjuvant-induced arthritis in mice. AAPS PharmSciTech 2010;11(2):859–869. doi:https://doi.org/10.1208/s12249-010-9445-5.
Geary SM, Hu Q, Joshi VB, Bowden NB, Salem AK. Diaminosulfide based polymer microparticles as cancer vaccine delivery systems. J Control Release. 2015;220(Pt B):682–90. https://doi.org/10.1016/j.jconrel.2015.09.002.
Intra J, Salem AK. Fabrication, characterization and in vitro evaluation of poly(D,L-lactide-co-glycolide) microparticles loaded with polyamidoamine-plasmid DNA dendriplexes for applications in nonviral gene delivery. J Pharm Sci. 2010;99(1):368–84. https://doi.org/10.1002/jps.21840.
Lew B, Kim IY, Choi H, Kim KK. Sustained exenatide delivery via intracapsular microspheres for improved survival and function of microencapsulated porcine islets. Drug Deliv Transl Res. 2018;8(3):857–62. https://doi.org/10.1007/s13346-018-0484-x.
Ansary RH, Rahman MM, Awang MB, Katas H, Hadi H, Doolaanea AA. Preparation, characterization, and in vitro release studies of insulin-loaded double-walled poly(lactide-co-glycolide) microspheres. Drug Deliv Transl Res. 2016;6(3):308–18. https://doi.org/10.1007/s13346-016-0278-y.
Andhariya JV, Shen J, Choi S, Wang Y, Zou Y, Burgess DJ. Development of in vitro-in vivo correlation of parenteral naltrexone loaded polymeric microspheres. J Control Release. 2017;255:27–35. https://doi.org/10.1016/j.jconrel.2017.03.396.
Naguib YW, Lansakara PD, Lashinger LM, Rodriguez BL, Valdes S, Niu M, et al. Synthesis, characterization, and in vitro and in vivo evaluations of 4-(N)-Docosahexaenoyl 2′, 2′-Difluorodeoxycytidine with potent and broad-spectrum antitumor activity. Neoplasia. 2016;18(1):33–48. https://doi.org/10.1016/j.neo.2015.11.012.
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft excel. Comput Methods Prog Biomed. 2010;99(3):306–14. https://doi.org/10.1016/j.cmpb.2010.01.007.
Yao J, Qian C, Shu T, Zhang X, Zhao Z, Liang Y. Combination treatment of PD98059 and DAPT in gastric cancer through induction of apoptosis and downregulation of WNT/beta-catenin. Cancer Biol Ther. 2013;14(9):833–9. https://doi.org/10.4161/cbt.25332.
Awasthi N, Monahan S, Stefaniak A, Schwarz MA, Schwarz RE. Inhibition of the MEK/ERK pathway augments nab-paclitaxel-based chemotherapy effects in preclinical models of pancreatic cancer. Oncotarget. 2018;9(4):5274–86. https://doi.org/10.18632/oncotarget.23684.
Cilurzo F, Selmin F, Minghetti P, Montanari L. Design of methylprednisolone biodegradable microspheres intended for intra-articular administration. AAPS PharmSciTech. 2008;9(4):1136–42. https://doi.org/10.1208/s12249-008-9158-1.
Zhang H, Gao S. Temozolomide/PLGA microparticles and antitumor activity against glioma C6 cancer cells in vitro. Int J Pharm. 2007;329(1–2):122–8. https://doi.org/10.1016/j.ijpharm.2006.08.027.
Ogawa R, Stachnik JM, Echizen H. Clinical pharmacokinetics of drugs in patients with heart failure: an update (part 2, drugs administered orally). Clin Pharmacokinet. 2014;53(12):1083–114. https://doi.org/10.1007/s40262-014-0189-3.
Shammas FV, Dickstein K. Clinical pharmacokinetics in heart failure. An updated review. Clin Pharmacokinet. 1988;15(2):94–113. https://doi.org/10.2165/00003088-198815020-00002.
Nies AS. Clinical pharmacokinetics in congestive heart failure. In: Hosenpud JD, Greenberg BH, editors. Congestive heart failure: pathophysiology, diagnosis, and comprehensive approach to management. New York: Springer New York; 1994. p. 323–40.
Ariza-Andraca CR, Altamirano-Bustamante E, Frati-Munari AC, Altamirano-Bustamante P, Graef-Sanchez A. Delayed insulin absorption due to subcutaneous edema. Arch Invest Med. 1991;22(2):229–33.
Navas JP, Martinez-Maldonado M. Pathophysiology of edema in congestive heart failure. Heart Dis Stroke. 1993;2(4):325–9.
Acknowledgements
Graphical abstract was designed using mindthegraph.com.
Funding
This work was supported by the National Institute of Health grants R01-HL-136149 to R.B.F. and S10-OD-019941 to R.M.W., and by the Lyle and Sharon Bighley Professorship (A.K.S.). B.E.G. was supported by the National GEM Consortium, the Alfred P. Sloan Minority Ph.D. Scholarship and the University of Iowa Graduate College Dean’s Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naguib, Y.W., Givens, B.E., Ho, G. et al. An injectable microparticle formulation for the sustained release of the specific MEK inhibitor PD98059: in vitro evaluation and pharmacokinetics. Drug Deliv. and Transl. Res. 11, 182–191 (2021). https://doi.org/10.1007/s13346-020-00758-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00758-9
Keywords
Profiles
- Brittany E. Givens View author profile