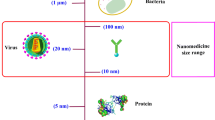
Abstract
After the discovery of the enhanced permeability and retention effect in 1986, it was envisioned that nanoparticle-mediated tumor-targeted delivery of chemotherapeutics would make a radical change in cancer therapy. However, after three decades of extensive research, only a few nanotherapeutics have been approved for clinical use. Although significant advantages of nanomedicines have been demonstrated in pre-clinical studies, clinical outcome was found to be variable. Advanced research has revealed that significant biochemical and structural variations exist between (and among) different tumors. These variations can considerably affect the tumor delivery and efficacy of nanomedicines. Tumor penetration is an important determining factor for positive therapeutic outcome and same nanomedicine can show diverse efficacy against different tumors depending on the extent of tumor accumulation and penetration. Recent research has started shading light on how the tumor variations can influence nanoparticle tumor delivery. These findings indicate that there is no “ideal” design of nanoparticles for exhibiting equally high efficacy against a broad spectrum of tumors. For achieving maximum benefit of the nanotherapeutics, it is necessary to analyze the tumor microenvironment for understanding the biological and structural characteristics of the tumor. Designing of the nanomedicine should be done according to the tumor characteristics. In this comprehensive review, we have first given a brief overview of the design characteristics of nanomedicine which impact their tumor delivery. Then we discussed about the variability in the tumor architecture and how it influences nanomedicine delivery. Finally, we have discussed the possibility of delivery system personalization based on the tumor characteristics.





Similar content being viewed by others
References
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92.
Singh R, Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–23. https://doi.org/10.1016/j.yexmp.2008.12.004.
Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25–37. https://doi.org/10.1007/978-1-60761-609-2_3.
Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978;38(9):2651–60.
Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. https://doi.org/10.1038/nature11412.
Li Y, Wang J, Wientjes MG, Au JL. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev. 2012;64(1):29–39. https://doi.org/10.1016/j.addr.2011.04.006.
Nichols JW, Bae YH. EPR: evidence and fallacy. J Control Release. 2014;190:451–64. https://doi.org/10.1016/j.jconrel.2014.03.057.
McNeil SE, (2009) Nanoparticle therapeutics: a personal perspective. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 1 (3):264–271
Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–27. https://doi.org/10.1038/nrd2591.
Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318.
Roy A, Ernsting MJ, Undzys E, Li SD. A highly tumor-targeted nanoparticle of podophyllotoxin penetrated tumor core and regressed multidrug resistant tumors. Biomaterials. 2015;52:335–46. https://doi.org/10.1016/j.biomaterials.2015.02.041.
Yang Y, Roy A, Zhao Y, Undzys E, Li SD. Comparison of tumor penetration of podophyllotoxin-carboxymethylcellulose conjugates with various chemical compositions in tumor spheroid culture and in vivo solid tumor. Bioconjug Chem. 2017;28(5):1505–18. https://doi.org/10.1021/acs.bioconjchem.7b00165.
Ernsting MJ, Murakami M, Roy A, Li SD. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172(3):782–94. https://doi.org/10.1016/j.jconrel.2013.09.013.
Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377(Pt 1):159–69. https://doi.org/10.1042/BJ20031253.
Tammam SN, Azzazy HME, Lamprecht A. The effect of nanoparticle size and NLS density on nuclear targeting in cancer and normal cells; impaired nuclear import and aberrant nanoparticle intracellular trafficking in glioma. J Control Release. 2017;253:30–6. https://doi.org/10.1016/j.jconrel.2017.02.029.
Tammam SN, Azzazy HM, Breitinger HG, Lamprecht A. Chitosan nanoparticles for nuclear targeting: the effect of nanoparticle size and nuclear localization sequence density. Mol Pharm. 2015;12(12):4277–89. https://doi.org/10.1021/acs.molpharmaceut.5b00478.
Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26(1):244–9. https://doi.org/10.1007/s11095-008-9626-z.
Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4. https://doi.org/10.1073/pnas.0600997103.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51. https://doi.org/10.1038/nbt.3330.
Xie X, Liao J, Shao X, Li Q, Lin Y. The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles. Sci Rep. 2017;7(1):3827. https://doi.org/10.1038/s41598-017-04229-z.
Levchenko TS, Rammohan R, Lukyanov AN, Whiteman KR, Torchilin VP. Liposome clearance in mice: the effect of a separate and combined presence of surface charge and polymer coating. Int J Pharm. 2002;240(1–2):95–102.
Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32(13):3435–46. https://doi.org/10.1016/j.biomaterials.2011.01.021.
Stylianopoulos T, Poh MZ, Insin N, Bawendi MG, Fukumura D, Munn LL, et al. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys J. 2010;99(5):1342–9. https://doi.org/10.1016/j.bpj.2010.06.016.
Lieleg O, Baumgartel RM, Bausch AR. Selective filtering of particles by the extracellular matrix: an electrostatic bandpass. Biophys J. 2009;97(6):1569–77. https://doi.org/10.1016/j.bpj.2009.07.009.
Bannunah AM, Vllasaliu D, Lord J, Stolnik S. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: effect of size and surface charge. Mol Pharm. 2014;11(12):4363–73. https://doi.org/10.1021/mp500439c.
Rao L, Meng QF, Bu LL, Cai B, Huang Q, Sun ZJ, et al. Erythrocyte membrane-coated upconversion nanoparticles with minimal protein adsorption for enhanced tumor imaging. ACS Appl Mater Interfaces. 2017;9(3):2159–68. https://doi.org/10.1021/acsami.6b14450.
Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–5. https://doi.org/10.1073/pnas.1106634108.
Yokoe J, Sakuragi S, Yamamoto K, Teragaki T, Ogawara K, Higaki K, et al. Albumin-conjugated PEG liposome enhances tumor distribution of liposomal doxorubicin in rats. Int J Pharm. 2008;353(1–2):28–34. https://doi.org/10.1016/j.ijpharm.2007.11.008.
Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016;1:16014. https://doi.org/10.1038/natrevmats.2016.14. https://www.nature.com/articles/natrevmats201614#supplementary-information
Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134(4):2139–47. https://doi.org/10.1021/ja2084338.
Dos Santos N, Allen C, Doppen AM, Anantha M, Cox KA, Gallagher RC, et al. Influence of poly (ethylene glycol) grafting density and polymer length on liposomes: relating plasma circulation lifetimes to protein binding. Biochim Biophys Acta. 2007;1768(6):1367–77. https://doi.org/10.1016/j.bbamem.2006.12.013.
Sengupta S. Cancer nanomedicine: lessons for immuno-oncology. Trends in cancer. 2017;3(8):551–60. https://doi.org/10.1016/j.trecan.2017.06.006.
Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145(3):178–81. https://doi.org/10.1016/j.jconrel.2010.03.016.
Tagami T, Nakamura K, Shimizu T, Yamazaki N, Ishida T, Kiwada H. CpG motifs in pDNA-sequences increase anti-PEG IgM production induced by PEG-coated pDNA-lipoplexes. J Control Release. 2010;142(2):160–6. https://doi.org/10.1016/j.jconrel.2009.10.017.
Hak S, Helgesen E, Hektoen HH, Huuse EM, Jarzyna PA, Mulder WJ, et al. The effect of nanoparticle polyethylene glycol surface density on ligand-directed tumor targeting studied in vivo by dual modality imaging. ACS Nano. 2012;6(6):5648–58. https://doi.org/10.1021/nn301630n.
Zhu X, Tao W, Liu D, Wu J, Guo Z, Ji X, et al. Surface de-PEGylation controls nanoparticle-mediated siRNA delivery in vitro and in vivo. Theranostics. 2017;7(7):1990–2002. https://doi.org/10.7150/thno.18136.
Kim HK, Van den Bossche J, Hyun SH, Thompson DH. Acid-triggered release via dePEGylation of fusogenic liposomes mediated by heterobifunctional phenyl-substituted vinyl ethers with tunable pH-sensitivity. Bioconjug Chem. 2012;23(10):2071–7. https://doi.org/10.1021/bc300266y.
Xu H, Deng Y, Chen D, Hong W, Lu Y, Dong X. Esterase-catalyzed dePEGylation of pH-sensitive vesicles modified with cleavable PEG-lipid derivatives. J Control Release. 2008;130(3):238–45. https://doi.org/10.1016/j.jconrel.2008.05.009.
LoPresti C, Massignani M, Fernyhough C, Blanazs A, Ryan AJ, Madsen J, et al. Controlling polymersome surface topology at the nanoscale by membrane confined polymer/polymer phase separation. ACS Nano. 2011;5(3):1775–84. https://doi.org/10.1021/nn102455z.
Niu Y, Yu M, Hartono SB, Yang J, Xu H, Zhang H, et al. Nanoparticles mimicking viral surface topography for enhanced cellular delivery. Adv Mater. 2013;25(43):6233–7. https://doi.org/10.1002/adma.201302737.
Wurster EC, Liebl R, Michaelis S, Robelek R, Wastl DS, Giessibl FJ, et al. Oligolayer-coated nanoparticles: impact of surface topography at the nanobio interface. ACS Appl Mater Interfaces. 2015;7(15):7891–900. https://doi.org/10.1021/am508435j.
Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141(5):769–84. https://doi.org/10.1007/s00432-014-1767-3.
Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3(5):269–80. https://doi.org/10.1038/ncponc0509.
Harding J, Burtness B. Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody. Drugs Today. 2005;41(2):107–27. https://doi.org/10.1358/dot.2005.41.2.882662.
Hogemann-Savellano D, Bos E, Blondet C, Sato F, Abe T, Josephson L, et al. The transferrin receptor: a potential molecular imaging marker for human cancer. Neoplasia. 2003;5(6):495–506.
Schmidt LH, Heitkotter B, Schulze AB, Schliemann C, Steinestel K, Trautmann M, et al. Prostate specific membrane antigen (PSMA) expression in non-small cell lung cancer. PLoS One. 2017;12(10):e0186280. https://doi.org/10.1371/journal.pone.0186280.
Cirstoiu-Hapca A, Bossy-Nobs L, Buchegger F, Gurny R, Delie F. Differential tumor cell targeting of anti-HER2 (Herceptin) and anti-CD20 (Mabthera) coupled nanoparticles. Int J Pharm. 2007;331(2):190–6. https://doi.org/10.1016/j.ijpharm.2006.12.002.
Ni X, Castanares M, Mukherjee A, Lupold SE. Nucleic acid aptamers: clinical applications and promising new horizons. Curr Med Chem. 2011;18(27):4206–14.
Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103(16):6315–20. https://doi.org/10.1073/pnas.0601755103.
Parashar A. Aptamers in therapeutics. J Clin Diagn Res. 2016;10(6):BE01–6. https://doi.org/10.7860/JCDR/2016/18712.7922.
Sahoo SK, Ma W, Labhasetwar V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int J Cancer. 2004;112(2):335–40. https://doi.org/10.1002/ijc.20405.
Zhang J, Wang L, Fai Chan H, Xie W, Chen S, He C, et al. Co-delivery of paclitaxel and tetrandrine via iRGD peptide conjugated lipid-polymer hybrid nanoparticles overcome multidrug resistance in cancer cells. Sci Rep. 2017;7:46057. https://doi.org/10.1038/srep46057.
Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J Pharm Sci. 2005;94(10):2135–46. https://doi.org/10.1002/jps.20457.
Better nanoparticle targeting with P-selectin. Cancer Discov. 2016;6(9):936. https://doi.org/10.1158/2159-8290.CD-NB2016-093.
Mizrachi A, Shamay Y, Shah J, Brook S, Soong J, Rajasekhar VK, et al. Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma. Nat Commun. 2017;8:14292. https://doi.org/10.1038/ncomms14292.
Doolittle E, Peiris PM, Doron G, Goldberg A, Tucci S, Rao S, et al. Spatiotemporal targeting of a dual-ligand nanoparticle to cancer metastasis. ACS Nano. 2015;9(8):8012–21. https://doi.org/10.1021/acsnano.5b01552.
Cheng H, Zhu JY, Xu XD, Qiu WX, Lei Q, Han K, et al. Activable cell-penetrating peptide conjugated prodrug for tumor targeted drug delivery. ACS Appl Mater Interfaces. 2015;7(29):16061–9. https://doi.org/10.1021/acsami.5b04517.
Cesbron Y, Shaheen U, Free P, Levy R. TAT and HA2 facilitate cellular uptake of gold nanoparticles but do not lead to cytosolic localisation. PLoS One. 2015;10(4):e0121683. https://doi.org/10.1371/journal.pone.0121683.
Trabulo S, Cardoso AL, Mano M, De Lima MC. Cell-penetrating peptides-mechanisms of cellular uptake and generation of delivery systems. Pharmaceuticals. 2010;3(4):961–93. https://doi.org/10.3390/ph3040961.
Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52(19):5144–53.
Lee H, Fonge H, Hoang B, Reilly RM, Allen C. The effects of particle size and molecular targeting on the intratumoral and subcellular distribution of polymeric nanoparticles. Mol Pharm. 2010;7(4):1195–208. https://doi.org/10.1021/mp100038h.
Ibrahim SA, Katara GK, Kulshrestha A, Jaiswal MK, Amin MA, Beaman KD. Breast cancer associated a2 isoform vacuolar ATPase immunomodulates neutrophils: potential role in tumor progression. Oncotarget. 2015;6(32):33033–45. https://doi.org/10.18632/oncotarget.5439.
Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med. 2016;94(2):155–71. https://doi.org/10.1007/s00109-015-1307-x.
Tan S, Wang G. Redox-responsive and pH-sensitive nanoparticles enhanced stability and anticancer ability of erlotinib to treat lung cancer in vivo. Drug Des Devel Ther. 2017;11:3519–29. https://doi.org/10.2147/DDDT.S151422.
Lv Y, Hao L, Hu W, Ran Y, Bai Y, Zhang L. Novel multifunctional pH-sensitive nanoparticles loaded into microbubbles as drug delivery vehicles for enhanced tumor targeting. Sci Rep. 2016;6:29321. https://doi.org/10.1038/srep29321.
Li M, Tang Z, Lv S, Song W, Hong H, Jing X, et al. Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery of doxorubicin. Biomaterials. 2014;35(12):3851–64. https://doi.org/10.1016/j.biomaterials.2014.01.018.
Zhu L, Torchilin VP. Stimulus-responsive nanopreparations for tumor targeting. Integr Biol. 2013;5(1):96–107. https://doi.org/10.1039/c2ib20135f.
Xu R, Wang XL, Lu ZR. New amphiphilic carriers forming pH-sensitive nanoparticles for nucleic acid delivery. Langmuir. 2010;26(17):13874–82. https://doi.org/10.1021/la1024185.
Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12(5):323–34. https://doi.org/10.1038/nrc3261.
Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805(1):105–17. https://doi.org/10.1016/j.bbcan.2009.11.002.
Sun XX, Yu Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol Sin. 2015;36(10):1219–27. https://doi.org/10.1038/aps.2015.92.
Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. https://doi.org/10.1093/carcin/bgp220.
Shah M, Allegrucci C. Keeping an open mind: highlights and controversies of the breast cancer stem cell theory. Breast Cancer. 2012;4:155–66. https://doi.org/10.2147/BCTT.S26434.
Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–92. https://doi.org/10.1016/j.cell.2007.01.029.
Illmensee K, Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci U S A. 1976;73(2):549–53.
Mahdipour-Shirayeh A, Kaveh K, Kohandel M, Sivaloganathan S. Phenotypic heterogeneity in modeling cancer evolution. PLoS One. 2017;12(10):e0187000. https://doi.org/10.1371/journal.pone.0187000.
Roy A, Li SD. Modifying the tumor microenvironment using nanoparticle therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(6):891–908. https://doi.org/10.1002/wnan.1406.
Mikhail AS, Allen C. Block copolymer micelles for delivery of cancer therapy: transport at the whole body, tissue and cellular levels. J Control Release. 2009;138(3):214–23. https://doi.org/10.1016/j.jconrel.2009.04.010.
Li L, Sun J, He Z. Deep penetration of nanoparticulate drug delivery systems into tumors: challenges and solutions. Curr Med Chem. 2013;20(23):2881–91.
Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6(12):815–23. https://doi.org/10.1038/nnano.2011.166.
Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–6.
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–8. https://doi.org/10.1053/sonc.2002.37263.
Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161(2):175–87. https://doi.org/10.1016/j.jconrel.2011.09.063.
Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev. 2011;37(1):63–74. https://doi.org/10.1016/j.ctrv.2010.05.001.
de Jong M, Maina T. Of mice and humans: are they the same?—implications in cancer translational research. J Nucl Med. 2010;51(4):501–4. https://doi.org/10.2967/jnumed.109.065706.
Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60(5):1388–93.
Lammers T, Peschke P, Kuhnlein R, Subr V, Ulbrich K, Debus J, et al. Effect of radiotherapy and hyperthermia on the tumor accumulation of HPMA copolymer-based drug delivery systems. J Control Release. 2007;117(3):333–41. https://doi.org/10.1016/j.jconrel.2006.10.032.
Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology. 2005;7(4):452–64. https://doi.org/10.1215/S1152851705000232.
Zhang L, Nishihara H, Kano MR. Pericyte-coverage of human tumor vasculature and nanoparticle permeability. Biol Pharm Bull. 2012;35(5):761–6.
Kano MR, Komuta Y, Iwata C, Oka M, Shirai YT, Morishita Y, et al. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. 2009;100(1):173–80. https://doi.org/10.1111/j.1349-7006.2008.01003.x.
Kano MR. Nanotechnology and tumor microcirculation. Adv Drug Deliv Rev. 2014;74:2–11. https://doi.org/10.1016/j.addr.2013.08.010.
Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Rel. 2016;244(Pt A):108–21. https://doi.org/10.1016/j.jconrel.2016.11.015.
Achilles EG, Fernandez A, Allred EN, Kisker O, Udagawa T, Beecken WD, et al. Heterogeneity of angiogenic activity in a human liposarcoma: a proposed mechanism for “no take” of human tumors in mice. J Natl Cancer Inst. 2001;93(14):1075–81.
Yu JL, Rak JW, Carmeliet P, Nagy A, Kerbel RS, Coomber BL. Heterogeneous vascular dependence of tumor cell populations. Am J Pathol. 2001;158(4):1325–34. https://doi.org/10.1016/S0002-9440(10)64083-7.
Choi M, Choi K, Ryu SW, Lee J, Choi C. Dynamic fluorescence imaging for multiparametric measurement of tumor vasculature. J Biomed Opt. 2011;16(4):046008. https://doi.org/10.1117/1.3562956.
Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–64. https://doi.org/10.1038/nrclinonc.2010.139.
Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92. https://doi.org/10.2147/HP.S93413.
Lee H, Hoang B, Fonge H, Reilly RM, Allen C. In vivo distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharm Res. 2010;27(11):2343–55. https://doi.org/10.1007/s11095-010-0068-z.
Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014;33(14):1743–54. https://doi.org/10.1038/onc.2013.121.
Miao L, Huang L. Exploring the tumor microenvironment with nanoparticles. Cancer Treat Res. 2015;166:193–226. https://doi.org/10.1007/978-3-319-16555-4_9.
Brown EB, Boucher Y, Nasser S, Jain RK. Measurement of macromolecular diffusion coefficients in human tumors. Microvasc Res. 2004;67(3):231–6. https://doi.org/10.1016/j.mvr.2004.02.001.
Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497–503.
Sykes EA, Dai Q, Sarsons CD, Chen J, Rocheleau JV, Hwang DM, et al. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc Natl Acad Sci U S A. 2016;113(9):E1142–51. https://doi.org/10.1073/pnas.1521265113.
Yokoi K, Kojic M, Milosevic M, Tanei T, Ferrari M, Ziemys A. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer Res. 2014;74(16):4239–46. https://doi.org/10.1158/0008-5472.CAN-13-3494.
Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–33. https://doi.org/10.1038/nrc1094.
Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163(5):1801–15. https://doi.org/10.1016/S0002-9440(10)63540-7.
Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296(5574):1883–6. https://doi.org/10.1126/science.1071420.
Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–13. https://doi.org/10.1038/nrc1456.
Hompland T, Ellingsen C, Ovrebo KM, Rofstad EK. Interstitial fluid pressure and associated lymph node metastasis revealed in tumors by dynamic contrast-enhanced MRI. Cancer Res. 2012;72(19):4899–908. https://doi.org/10.1158/0008-5472.CAN-12-0903.
Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cellular & molecular immunology. 2015;12(1):1–4. https://doi.org/10.1038/cmi.2014.83.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. https://doi.org/10.1016/j.cell.2010.03.014.
Kubota K, Moriyama M, Furukawa S, Rafiul H, Maruse Y, Jinno T, et al. CD163(+)CD204(+) tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci Rep. 2017;7(1):1755. https://doi.org/10.1038/s41598-017-01661-z.
Cuccarese MF, Dubach JM, Pfirschke C, Engblom C, Garris C, Miller MA, et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat Commun. 2017;8:14293. https://doi.org/10.1038/ncomms14293.
Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, et al. The prognostic and clinicopathological significance of tumor-associated macrophages in patients with gastric cancer: a meta-analysis. PLoS One. 2017;12(1):e0170042. https://doi.org/10.1371/journal.pone.0170042.
Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2. https://doi.org/10.1038/npjbcancer.2015.25.
Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, et al. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. Journal of pathology and translational medicine. 2015;49(4):318–24. https://doi.org/10.4132/jptm.2015.06.01.
Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228(7):1404–12. https://doi.org/10.1002/jcp.24260.
Chen XW, Yu TJ, Zhang J, Li Y, Chen HL, Yang GF, et al. CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene. 2017;36(35):5045–57. https://doi.org/10.1038/onc.2017.118.
Qing W, Fang WY, Ye L, Shen LY, Zhang XF, Fei XC, et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. 2012;22(9):905–10. https://doi.org/10.1089/thy.2011.0452.
Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105(1):1–8. https://doi.org/10.1111/cas.12314.
Shaffer SA, Baker-Lee C, Kennedy J, Lai MS, de Vries P, Buhler K, et al. In vitro and in vivo metabolism of paclitaxel poliglumex: identification of metabolites and active proteases. Cancer Chemother Pharmacol. 2007;59(4):537–48. https://doi.org/10.1007/s00280-006-0296-4.
Jackson EF, Esparza-Coss E, Wen X, Ng CS, Daniel SL, Price RE, et al. Magnetic resonance imaging of therapy-induced necrosis using gadolinium-chelated polyglutamic acids. Int J Radiat Oncol Biol Phys. 2007;68(3):830–8. https://doi.org/10.1016/j.ijrobp.2007.01.011.
Alizadeh D, Zhang L, Hwang J, Schluep T, Badie B. Tumor-associated macrophages are predominant carriers of cyclodextrin-based nanoparticles into gliomas. Nanomedicine. 2010;6(2):382–90. https://doi.org/10.1016/j.nano.2009.10.001.
Penn CA, Yang K, Zong H, Lim JY, Cole A, Yang D, et al. Therapeutic impact of nanoparticle therapy targeting tumor-associated macrophages. Mol Cancer Ther. 2018;17(1):96–106. https://doi.org/10.1158/1535-7163.MCT-17-0688.
Zamboni WC, Eiseman JL, Strychor S, Rice PM, Joseph E, Zamboni BA, et al. Tumor disposition of pegylated liposomal CKD-602 and the reticuloendothelial system in preclinical tumor models. J Liposome Res. 2011;21(1):70–80. https://doi.org/10.3109/08982101003754385.
Zhu S, Niu M, O'Mary H, Cui Z. Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Mol Pharm. 2013;10(9):3525–30. https://doi.org/10.1021/mp400216r.
Qiu W, Su GH. Development of orthotopic pancreatic tumor mouse models. Methods Mol Biol. 2013;980:215–23. https://doi.org/10.1007/978-1-62703-287-2_11.
Morton JJ, Bird G, Keysar SB, Astling DP, Lyons TR, Anderson RT, et al. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene. 2016;35(3):290–300. https://doi.org/10.1038/onc.2015.94.
Pompili L, Porru M, Caruso C, Biroccio A, Leonetti C. Patient-derived xenografts: a relevant preclinical model for drug development. J Exp Clin Cancer Res. 2016;35(1):189. https://doi.org/10.1186/s13046-016-0462-4.
DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17(11):1514–20. https://doi.org/10.1038/nm.2454.
Rangarajan A, Weinberg RA. Opinion: comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2003;3(12):952–9. https://doi.org/10.1038/nrc1235.
Politi K, Pao W. How genetically engineered mouse tumor models provide insights into human cancers. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(16):2273–81. https://doi.org/10.1200/JCO.2010.30.8304.
Cekanova M, Rathore K. Animal models and therapeutic molecular targets of cancer: utility and limitations. Drug Des Devel Ther. 2014;8:1911–21. https://doi.org/10.2147/DDDT.S49584.
Garralda E, Paz K, Lopez-Casas PP, Jones S, Katz A, Kann LM, et al. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin Cancer Res. 2014;20(9):2476–84. https://doi.org/10.1158/1078-0432.CCR-13-3047.
Song G, Suzuki OT, Santos CM, Lucas AT, Wiltshire T, Zamboni WC. Gulp1 is associated with the pharmacokinetics of PEGylated liposomal doxorubicin (PLD) in inbred mouse strains. Nanomedicine. 2016;12(7):2007–17. https://doi.org/10.1016/j.nano.2016.05.019.
Miller MA, Chandra R, Cuccarese MF, Pfirschke C, Engblom C, Stapleton S, et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci Transl Med. 2017;9(392):eaal0225. https://doi.org/10.1126/scitranslmed.aal0225.
Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–63. https://doi.org/10.1016/j.ccr.2004.10.011.
Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–6. https://doi.org/10.1158/0008-5472.CAN-04-0074.
Shenoi MM, Iltis I, Choi J, Koonce NA, Metzger GJ, Griffin RJ, et al. Nanoparticle delivered vascular disrupting agents (VDAs): use of TNF-alpha conjugated gold nanoparticles for multimodal cancer therapy. Mol Pharm. 2013;10(5):1683–94. https://doi.org/10.1021/mp300505w.
Langer CJ, O'Byrne KJ, Socinski MA, Mikhailov SM, Lesniewski-Kmak K, Smakal M, et al. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2008;3(6):623–30. https://doi.org/10.1097/JTO.0b013e3181753b4b.
Melancon MP, Wang W, Wang Y, Shao R, Ji X, Gelovani JG, et al. A novel method for imaging in vivo degradation of poly (L-glutamic acid), a biodegradable drug carrier. Pharm Res. 2007;24(6):1217–24. https://doi.org/10.1007/s11095-007-9253-0.
Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev. 2017;108:25–38. https://doi.org/10.1016/j.addr.2016.04.025.
Stapleton S, Allen C, Pintilie M, Jaffray DA. Tumor perfusion imaging predicts the intra-tumoral accumulation of liposomes. J Control Release. 2013;172(1):351–7. https://doi.org/10.1016/j.jconrel.2013.08.296.
Karathanasis E, Suryanarayanan S, Balusu SR, McNeeley K, Sechopoulos I, Karellas A, et al. Imaging nanoprobe for prediction of outcome of nanoparticle chemotherapy by using mammography. Radiology. 2009;250(2):398–406. https://doi.org/10.1148/radiol.2502080801.
Yokoi K, Tanei T, Godin B, van de Ven AL, Hanibuchi M, Matsunoki A, et al. Serum biomarkers for personalization of nanotherapeutics-based therapy in different tumor and organ microenvironments. Cancer Lett. 2014;345(1):48–55. https://doi.org/10.1016/j.canlet.2013.11.015.
Wu J, Akaike T, Hayashida K, Okamoto T, Okuyama A, Maeda H. Enhanced vascular permeability in solid tumor involving peroxynitrite and matrix metalloproteinases. Jpn J Cancer Res. 2001;92(4):439–51.
Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. https://doi.org/10.1016/j.addr.2015.01.002.
Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD, Li SD. Docetaxel conjugate nanoparticles that target alpha-smooth muscle actin-expressing stromal cells suppress breast cancer metastasis. Cancer Res. 2013;73(15):4862–71. https://doi.org/10.1158/0008-5472.CAN-13-0062.
Hoang B, Ernsting MJ, Roy A, Murakami M, Undzys E, Li SD. Docetaxel-carboxymethylcellulose nanoparticles target cells via a SPARC and albumin dependent mechanism. Biomaterials. 2015;59:66–76. https://doi.org/10.1016/j.biomaterials.2015.04.032.
Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’—more than meets the eye. Trends Mol Med. 2013;19(8):447–53. https://doi.org/10.1016/j.molmed.2013.05.004.
Ernsting MJ, Hoang B, Lohse I, Undzys E, Cao P, Do T, et al. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J Control Release. 2015;206:122–30. https://doi.org/10.1016/j.jconrel.2015.03.023.
Park K. The drug delivery field at the inflection point: time to fight its way out of the egg. Journal of controlled release: official journal of the Controlled Release Society. 2017;267:2–14. https://doi.org/10.1016/j.jconrel.2017.07.030.
Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9(1):1410. https://doi.org/10.1038/s41467-018-03705-y.
Autio KA, Dreicer R, Anderson J, Garcia JA, Alva A, Hart LL, et al. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 2018; https://doi.org/10.1001/jamaoncol.2018.2168.
Raemdonck K, De Smedt SC. Lessons in simplicity that should shape the future of drug delivery. Nat Biotechnol. 2015;33(10):1026–7. https://doi.org/10.1038/nbt.3366.
Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018:JCO2017776112. https://doi.org/10.1200/JCO.2017.77.6112.
Funding
Both the authors thank the “Department of Science and Technology (DST), Govt. of India” for the financial support under the project ECR/2016/000566/LS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Swetha, K.L., Roy, A. Tumor heterogeneity and nanoparticle-mediated tumor targeting: the importance of delivery system personalization. Drug Deliv. and Transl. Res. 8, 1508–1526 (2018). https://doi.org/10.1007/s13346-018-0578-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-0578-5