Abstract
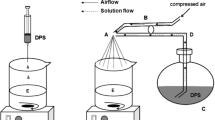
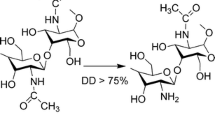
The aim of this study was the investigation of powder-based formulations for nasal administration of tacrine hydrochloride. The anti-Alzheimer drug was encapsulated in mucoadhesive microparticles based on chitosan/pectin polyelectrolyte complexes. Microparticles were prepared by means of two different technological approaches (direct spray-drying and spray-drying followed by lyophilization) and analysed in terms of size, morphology and physico-chemical characteristics. Moreover, water uptake and mucoadhesion ability were evaluated as well as drug release and permeation behaviour. The results suggest that lyophilization favours the formation of small particle aggregates with a size of 10 μm, instead of single particles (size smaller than 5 μm) such as direct spray-drying. Particles obtained with the two loading methods present different functional properties according to the different physical state of the loaded drug and its possible interaction with chitosan/pectin complex. Moreover, the presence of different amount of chitosan and pectin in the complex influences their ability to hydrate, interact with mucin and favour drug permeation.








Similar content being viewed by others
References
Maurya SK, Pathak K, Bali V. Therapeutic potential of mucoadhesive drug delivery systems—an updated patent review. Recent Patents Drug Deliv Formul. 2010;4(3):256–65. Review.
Alhalaweh A, Vilinska A, Gavini E, Rassu G, Velaga SP. Surface thermodynamics of mucoadhesive dry powder formulation of zolmitriptan. AAPS Pharm Sci Tech. 2011;12(4):1186–92.
Gavini E, Rassu G, Ferraro L, Generosi A, Rau JV, Brunetti A, et al. Influence of chitosan glutamate on the in vivo intranasal absorption of rokitamycin from microspheres. J Pharm Sci. 2010. doi:10.1002/jps.22382.
Bowey K, Neufeld RJ. Systemic and mucosal delivery of drugs within polymeric microparticles produced by spray drying. BioDrugs. 2010;24(6):359–77. Review.
Sollohub K, Cal K. Spray drying technique: II. Current applications in pharmaceutical technology. J Pharm Sci. 2010;99(2):587–97. Review.
Illum L. Nasal drug delivery—recent developments and future prospects. J Control Release. 2012. doi:10.1016/j.jconrel.2012.01.024.
Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337(1-2):1–24. Review.
Schroeter JD, Kimbell JS, Asgharian B. Analysis of particle deposition in the turbinate and olfactory region using a human nasal computational fluid dynamic model. J Aerosol Med. 2006;19:301–13.
Merkus FW, Verhoef JC, Schipper NG, Marttin E. Nasal mucociliary clearance as a factor for nasal drug delivery. Adv Drug Deliv Rev. 1998;29:13–38.
Callens C, Ceulemans J, Ludwig A, Foreman P, Remon JP. Rheological study on mucoadhesivity of some nasal powder formulations. Eur J Pharm Biopharm. 2003;55(3):323–8.
Hägerström H, Paulsson M, Edsman K. Evaluation of mucoadhesion for two polyelectrolyte gels in simulated physiological conditions using a rheological method. Eur J Pharm Biopharm. 2000;9(3):301–9.
Lai SK, Wang Y-Y, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61(2):86–100.
Morris G, Kök S, Harding S, Adams G. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol Genet Eng Rev. 2010;27:257–84. Review.
Hamman JH. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar Drugs. 2010;8(4):1305–22. Review.
Freeman SE, Dawson RM. Tacrine: a pharmacological review. Prog Neurobiol. 1991;36:257–77.
Ping H, Davis SS, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm. 1998;166:75–88.
Luppi B, Bigucci F, Abruzzo A, Corace G, Cerchiara T, Zecchi V. Freeze-dried chitosan/pectin nasal inserts for antipsychotic drug delivery. Eur J Pharm Biopharm. 2010;75:381–7.
Sorrenti M, Catenacci L, Bruni G, Luppi B, Bigucci F, Bettinetti G. Solid-state characterization of tacrine hydrochloride. J Pharm Biomed Anal. 2012;63:53–61.
Björk E, Isaksson U, Edman P, Artursson P. Starch microspheres induce pulsatile delivery of drugs and peptides across the epithelial barrier by reversible separation of the tight junctions. J Drug Target. 1995;2(6):501–7.
Cal K, Sollohub K. Spray drying technique. I: Hardware and process parameters. J Pharm Sci. 2010;99(2):575–86. Review.
Gavini E, Hegge AB, Rassu G, Sanna V, Testa C, Pirisino G, et al. Nasal administration of carbamazepine using chitosan microspheres: in vitro/in vivo studies. Int J Pharm. 2006;307(1):9–15.
Colombo P, Buttini F, Sonvico F, Colombo G, Russo P, Scatturin A, et al. Respiratory drug delivery. Proc Int Sch Adv Mater Sci Technol. 2005; 91–110.
Djupesland PG, Skretting A, Winderen M, Holand T. Bi-directional nasal delivery of aerosols can prevent lung deposition. J Aerosol Med. 2004;17(3):249–59.
Bigucci F, Luppi B, Monaco L, Cerchiara T, Zecchi V. Pectin-based microspheres for colon-specific delivery of vancomycin. J Pharm Pharmacol. 2009;61(1):41–6.
Luppi B, Bigucci F, Cerchiara T, Zecchi V. Chitosan-based hydrogels for nasal drug delivery: from inserts to nanoparticles. Expert Opin Drug Deliv. 2010;7(7):811–28.
Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials. 1996;17:1553–61.
Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Release. 1985;2:257–75.
Davis SS, Illum L. Absorption enhancers for nasal drug delivery. Clin Pharmacokinet. 2003;42(13):1107–28. Review.
Acknowledgments
This work was supported by a grant received from the Fondazione Cassa di Risparmio di Imola. The authors would like to thank Giulia Nerina Nardone for her contribution to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saladini, B., Bigucci, F., Cerchiara, T. et al. Microparticles based on chitosan/pectin polyelectrolyte complexes for nasal delivery of tacrine hydrochloride. Drug Deliv. and Transl. Res. 3, 33–41 (2013). https://doi.org/10.1007/s13346-012-0086-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-012-0086-y