Abstract
Background and Objectives
Finerenone (BAY 94-8862) is a selective, nonsteroidal mineralocorticoid receptor antagonist. The aim of this study was to assess the effect of mild or moderate hepatic impairment on the pharmacokinetics, safety and tolerability of finerenone.
Methods
The study was conducted in a single-center, nonrandomized, noncontrolled, nonblinded observational design with group stratification. A single oral 5-mg dose of finerenone was administered as a tablet to participants with mild or moderate hepatic impairment (Child–Pugh A, score 5–6 [n = 9], or Child–Pugh B, score 7–9 [n = 9], respectively) and to age-, weight- and sex-matched healthy participants (n = 9). The pharmacokinetics of finerenone and its metabolites were assessed in plasma and urine, and safety and tolerability were monitored.
Results
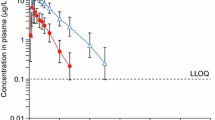
Finerenone area under the plasma concentration–time curve (AUC) and unbound AUC were 38% and 55% greater, respectively, in participants with moderate hepatic impairment than in healthy participants, whereas maximum plasma concentration (Cmax) was unchanged. No clear effects on AUC or Cmax were seen in participants with mild hepatic impairment. Finerenone was safe and well tolerated in all participants.
Conclusion
The effects of mild or moderate hepatic impairment on systemic exposure of finerenone are small, consistent with its low hepatic extraction and preponderance of gastrointestinal over hepatic first-pass clearance. Considering the small increases in AUC and the absence of changes in Cmax, a dose adaptation does not appear to be warranted in patients with mild or moderate hepatic impairment.




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65(2):257–63. https://doi.org/10.1161/HYPERTENSIONAHA.114.04488.
Schwenk MH, Hirsch JS, Bomback AS. Aldosterone blockade in CKD: emphasis on pharmacology. Adv Chronic Kidney Dis. 2015;22(2):123–32. https://doi.org/10.1053/j.ackd.2014.08.003.
Kolkhof P, Bärfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234(1):T125–40. https://doi.org/10.1530/JOE-16-0600.
Kolkhof P, Jaisser F, Kim SY, Filippatos G, Nowack C, Pitt B. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol. 2017;243:271–305. https://doi.org/10.1007/164_2016_76.
Bärfacker L, Kuhl A, Hillisch A, Grosser R, Figueroa-Perez S, Heckroth H, et al. Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7(8):1385–403. https://doi.org/10.1002/cmdc.201200081.
Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69–78. https://doi.org/10.1097/FJC.0000000000000091.
Kolkhof P, Borden SA. Molecular pharmacology of the mineralocorticoid receptor: prospects for novel therapeutics. Mol Cell Endocrinol. 2012;350(2):310–7. https://doi.org/10.1016/j.mce.2011.06.025.
Kolkhof P, Nowack C, Eitner F. Nonsteroidal antagonists of the mineralocorticoid receptor. Curr Opin Nephrol Hypertens. 2015;24(5):417–24. https://doi.org/10.1097/MNH.0000000000000147.
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884–94. https://doi.org/10.1001/jama.2015.10081.
Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, et al. A randomized controlled study of finerenone vs eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37(27):2105–14. https://doi.org/10.1093/eurheartj/ehw132.
Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453–63. https://doi.org/10.1093/eurheartj/eht187.
Lentini S, Heinig R, Kimmeskamp-Kirschbaum N, Wensing G. Pharmacokinetics, safety and tolerability of the novel, selective mineralocorticoid receptor antagonist finerenone – results from first-in-man and relative bioavailability studies. Fundam Clin Pharmacol. 2016;30(2):172–84. https://doi.org/10.1111/fcp.12170.
Heinig R, Kimmeskamp-Kirschbaum N, Halabi A, Lentini S. Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94-8862) in individuals with renal impairment. Clin Pharmacol Drug Dev. 2016;5(6):488–501. https://doi.org/10.1002/cpdd.263.
Heinig R, Gerisch M, Engelen A, Nagelschmitz J, Loewen S. Pharmacokinetics of the novel, selective, non-steroidal mineralocorticoid receptor antagonist finerenone in healthy volunteers: results from an absolute bioavailability study and drug–drug interaction studies in vitro and in vivo. Eur J Drug Metab Pharmacokinet. 2018. https://doi.org/10.1007/s13318-018-0483-9.
Gerisch M, Heinig R, Engelen A, Lang D, Kolkhof P, Radtke M et al. Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans, in vivo and in vitro. Drug Metab Dispos. 2018. https://doi.org/10.1124/dmd.118.083337.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. https://doi.org/10.1002/bjs.1800600817.
European Medicines Agency CfMPfHUC. Guideline on bioanalytical method validation. July 2011. https://www.ema.europa.eu/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf. Accessed Jan 2019.
U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). Guidance for industry: bioanalytical method validation. May 2018. https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf. Accessed Jan 2019.
Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64(12):1147–61. https://doi.org/10.1007/s00228-008-0553-z.
Liu Y, Boettcher MF, Schmidt A, Unger S, Halabi A, Brendel E, et al. Pharmacokinetics and safety of nifedipine GITS/candesartan fixed-dose combination in subjects with hepatic impairment. Int J Clin Pharmacol Ther. 2017;55(3):246–55. https://doi.org/10.5414/CP202700.
Holtbecker N, Fromm MF, Kroemer HK, Ohnhaus EE, Heidemann H. The nifedipine-rifampin interaction. Evidence for induction of gut wall metabolism. Drug Metab Dispos. 1996;24(10):1121–3.
Albarmawi A, Czock D, Gauss A, Ehehalt R, Lorenzo Bermejo J, Burhenne J, et al. CYP3A activity in severe liver cirrhosis correlates with Child–Pugh and model for end-stage liver disease (MELD) scores. Br J Clin Pharmacol. 2014;77(1):160–9. https://doi.org/10.1111/bcp.12182.
Acknowledgements
Dr. Gabriele Rohde of Bayer AG (Wuppertal, Germany) was responsible for bioanalyses. Editorial assistance, funded by Bayer AG, was provided by Oxford PharmaGenesis Ltd, Oxford, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was funded by the sponsor, Bayer AG. Bayer AG agreed to the publication of the present data.
Conflict of interest
RH and JN are employees of Bayer AG. ML is an employee of CHRESTOS Concept GmbH & Co. KG, which received funding for this analysis from Bayer AG. AA and AH are employees of CRS Clinical Research Services Kiel GmbH, which received funding for study conduct from Bayer AG. In addition, RH and JN have stock in Bayer AG, but are not paid in stock or stock options.
Ethical approval
The study protocol (no. 14510) was reviewed and approved by the Independent Ethics Committee of the Medical Council of Schleswig–Holstein (Bad Segeberg, Germany). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants before study commencement.
Rights and permissions
About this article
Cite this article
Heinig, R., Lambelet, M., Nagelschmitz, J. et al. Pharmacokinetics of the Novel Nonsteroidal Mineralocorticoid Receptor Antagonist Finerenone (BAY 94-8862) in Individuals with Mild or Moderate Hepatic Impairment. Eur J Drug Metab Pharmacokinet 44, 619–628 (2019). https://doi.org/10.1007/s13318-019-00547-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-019-00547-x