Abstract
Background and Objectives
Tacrolimus is the mainstay of immunosuppression in renal transplantation. Given that once-daily administration improves patient compliance, 1:1 dose conversion from twice-daily Prograf® to once-daily Advagraf® is recommended. Although cytochrome P450 (CYP) 3A5 and multi-drug resistance 1 (MDR1) polymorphisms influence tacrolimus concentrations, it is unknown if these impact on conversion. This study investigated the change in the pharmacokinetics of tacrolimus after conversion from Prograf® to Advagraf® and examined the impact of CYP3A5 and MDR1 C3435T polymorphisms on those pharmacokinetics.
Methods
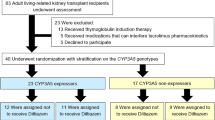
A prospective open-label pharmacokinetic study of 1:1 conversion from Prograf® to Advagraf® with or without diltiazem was conducted on 26 stable renal transplant recipients. Blood samples were collected over 24 h during each phase, tacrolimus concentrations were assayed, and noncompartmental pharmacokinetic analysis was performed. All participants were genotyped for CYP3A5*3 and MDR1 C3435T.
Results
After conversion, without diltiazem, the area under the concentration–time curve at steady state from 0 to 24 h after dose administration (AUCss, 0–24) was significantly reduced [median 224 (range 172–366) vs. 184 (104–347) ng·h/mL, p = 0.006, n = 26]. A decrease in tacrolimus exposure (median 21%) was only evident among CYP3A5 expressors [227 (172–366) vs. 180 (104–347) ng·h/mL, p = 0.014, n = 18], not among non-expressors [215 (197–290) vs. 217 (129–281) ng·h/mL, p = 0.263, n = 8]. In contrast, among CYP3A5 expressors receiving diltiazem, AUCss, 0–24 did not change significantly upon conversion [229 (170–296) vs. 221 (123–342) ng·h/mL, p = 0.575, n = 10]. An independent effect was not evident for MDR1 C3435T polymorphism.
Conclusion
The high prevalence of CYP3A5 polymorphism among Asians may lead to a significant reduction in tacrolimus exposure with 1:1 dose conversion of Prograf® to Advagraf®. These results advocate for CYP3A5 determination prior to conversion, and suggest that 1:1.25 conversion should be used for CYP3A5 expressors and 1:1 conversion for other patients.


Similar content being viewed by others
References
Astellas Pharma Canada, Inc. Prograf® product monograph. Markham: Astellas Pharma Canada, Inc.; 2011.
Astellas Pharma Canada, Inc. Advagraf® product monograph. Markham: Astellas Pharma Canada, Inc.; 2010.
Kuypers DR, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95(2):333–40.
European Medicines Agency. European public assessment report—scientific discussion paper on Advagraf. London: European Medicines Agency; 2007. https://www.ema.europa.eu/documents/scientific-discussion/advagraf-epar-scientific-discussion_en.pdf. Accessed 6 Nov 2018.
Barraclough KA, Isbel NM, Johnson DW, Campbell SB, Staatz CE. Once- versus twice-daily tacrolimus: are the formulations truly equivalent? Drugs. 2011;71(12):1561–77.
Alloway R, Steinberg S, Khalil K, et al. Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen. Transpl Proc. 2005;37(2):867–70.
Kurnatowska I, Krawczyk J, Oleksik T, Nowicki M. Tacrolimus dose and blood concentration variability in kidney transplant recipients undergoing conversion from twice daily to once daily modified release tacrolimus. Transpl Proc. 2011;43(8):2954–6.
Staatz CE, Tett SE. Clinical pharmacokinetics of once-daily tacrolimus in solid-organ transplant patients. Clin Pharmacokinet. 2015;54(10):993–1025.
Hooff J, Walt I, Kallmeyer J, et al. Pharmacokinetics in stable kidney transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. Ther Drug Monit. 2012;34(1):46–52.
Diez OB, Alonso AM, Aguado FS, Banos GM, Garcia MS, Gomez HE. Three-month experience with tacrolimus once-daily regimen in stable renal allografts. Transpl Proc. 2009;41(6):2323–5.
Hardinger KL, Park JM, Schnitzler MA, Koch MJ, Miller BW, Brennan DC. Pharmacokinetics of tacrolimus in kidney transplant recipients: twice daily versus once daily dosing. Am J Transplant. 2004;4(4):621–5.
Alloway R, Steinberg S, Khalil K, et al. Two years postconversion from a Prograf-based regimen to a once-daily tacrolimus extended-release formulation in stable kidney transplant recipients. Transplantation. 2007;83(12):1648–51.
Iaria G, Sforza D, Angelico R, et al. Switch from twice-daily tacrolimus (Prograf) to once-daily prolonged-release tacrolimus (Advagraf) in kidney transplantation. Transpl Proc. 2011;43(4):1028–9.
Press RR, de Fijter JW, Guchelaar HJ. Individualizing calcineurin inhibitor therapy in renal transplantation—current limitations and perspectives. Curr Pharm Des. 2010;16(2):176–86.
Lamba JK, Lin YS, Thummel K, et al. Common allelic variants of cytochrome P4503A4 and their prevalence in different populations. Pharmacogenetics. 2002;12(2):121–32.
Loh PT, Lou HX, Zhao Y, Chin YM, Vathsala A. Significant impact of gene polymorphisms on tacrolimus but not cyclosporine dosing in Asian renal transplant recipients. Transpl Proc. 2008;40(5):1690–5.
Tsuchiya N, Satoh S, Tada H, et al. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004;78(8):1182–7.
Glowacki F, Lionet A, Hammelin JP, et al. Influence of cytochrome P450 3A5 (CYP3A5) genetic polymorphism on the pharmacokinetics of the prolonged-release, once-daily formulation of tacrolimus in stable renal transplant recipients. Clin Pharmacokinet. 2011;50(7):451–9.
Li Y, Yan L, Shi Y, Bai Y, Tang J, Wang L. CYP3A5 and ABCB1 genotype influence tacrolimus and sirolimus pharmacokinetics in renal transplant recipients. SpringerPlus. 2015;4(1):637.
Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97(7):3473–8.
Anglicheau D, Verstuyft C, Laurent-Puig P, et al. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14(7):1889–96.
Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet. 2010;49(3):141–75.
Kothari J, Nash M, Zaltzman J, Ramesh Prasad GV. Diltiazem use in tacrolimus-treated renal transplant recipients. J Clin Pharm Ther. 2004;29(5):425–30.
Jones DR, Gorski JC, Hamman MA, Mayhew BS, Rider S, Hall SD. Diltiazem inhibition of cytochrome P-450 3A activity is due to metabolite intermediate complex formation. J Pharmacol Exp Ther. 1999;290(3):1116–25.
Li JL, Wang XD, Chen SY, et al. Effects of diltiazem on pharmacokinetics of tacrolimus in relation to CYP3A5 genotype status in renal recipients: from retrospective to prospective. Pharmacogenom J. 2011;11(4):300–6.
Ma MK, Kwan LP, Mok MM, Yap DY, Tang CS, Chan TM. Significant reduction of tacrolimus trough level after conversion from twice daily Prograf to once daily Advagraf in Chinese renal transplant recipients with or without concomitant diltiazem treatment. Ren Fail. 2013;35(7):942–5.
Wallemacq P, Goffinet JS, O’Morchoe S, et al. Multi-site analytical evaluation of the Abbott ARCHITECT tacrolimus assay. Ther Drug Monit. 2009;31(2):198–204.
Thervet E, Loriot MA, Barbier S, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87(6):721–6.
Shuker N, Bouamar R, van Schaik RH, et al. A randomized controlled trial comparing the efficacy of Cyp3a5 genotype-based with body-weight-based tacrolimus dosing after living donor kidney transplantation. Am J Transplant. 2016;16(7):2085–96.
Andrews LM, De Winter BC, Van Gelder T, Hesselink DA. Consideration of the ethnic prevalence of genotypes in the clinical use of tacrolimus. Pharmacogenomics. 2016;17(16):1737–40.
Andrews LM, Li Y, De Winter BCM, et al. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin Drug Metab Toxicol. 2017;13(12):1225–36.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Acknowledgements
The authors thank Ms. Tanusya Murali and the clinical care team for their assistance with patient recruitment, study coordination, and data collection. This study was presented in part at the 26th International Congress of The Transplantation Society (TTS 2016) in Hong Kong on 21 August 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by an Astellas Pharma Inc. Investigator Initiated Study Grant. Financial support included funding for: (1) genotyping of CYP3A5 rs776746 (CYP3A5*3) and MDR1 rs1045642 (MDR1 C3435T), (2) the trial coordinator, (3) pharmacokinetic studies, (4) standardized meals during pharmacokinetic studies, (5) Prograf® and Advagraf®, and (6) laboratory tests performed as part of the study protocol.
Conflict of interest
A. Vathsala has received honoraria from Astellas Pharma Inc. for talks given at scientific meetings. W.P. Yau and C.W.T. Loh declare that they have no conflict of interest.
Ethical approval
This study was approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB ref: 2012/01026) and granted a Clinical Trial Certificate (certificate number: CTC1300029) by the Health Sciences Authority, Singapore. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yau, WP., Loh, C.WT. & Vathsala, A. Conversion from Twice-Daily Prograf® to Once-Daily Advagraf® in Multi-ethnic Asian Adult Renal Transplant Recipients With or Without Concomitant Use of Diltiazem: Impact of CYP3A5 and MDR1 Genetic Polymorphisms on Tacrolimus Exposure. Eur J Drug Metab Pharmacokinet 44, 481–492 (2019). https://doi.org/10.1007/s13318-018-0531-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0531-5