Abstract
Background and Objectives
Monomethyl auristatin E (MMAE), the toxin linked to CD30-specific monoclonal antibody of Adcetris® (brentuximab vedotin), is a potent anti-microtubule agent. Brentuximab vedotin has been approved for the treatment of relapsed or refractory Hodgkin lymphoma and anaplastic large cell lymphoma. Cytochrome P450 (CYP) induction assessment of MMAE was conducted in human hepatocytes to assess DDI potentials and its translation to clinic.
Methods
MMAE was incubated at 1–1000 nM with cultured primary human hepatocytes for 72 h, and CYP1A2, CYP2B6, and CYP3A4 mRNA expression was assessed by quantitative reverse transcription-polymerase chain reaction and CYP-specific probe substrate by liquid chromatography coupled with mass spectrometry, along with microtubule disruption by immunofluorescence staining using anti-β-tubulin antibody and imaging.
Results
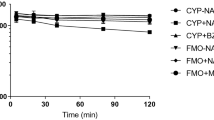
MMAE up to 10 nM had no significant effect on CYP1A2, CYP2B6, and CYP3A4 mRNA expression and activity, whereas at higher concentrations of 100- and 1000-nM MMAE, the CYP mRNA expression and activity were diminished substantially. Further investigation showed that the degree of CYP suppression was paralleled by that of microtubule disruption by MMAE, as measured by increase in the number of β-tubulin-positive aggregates. At the clinical dose, the concentration of MMAE was 7 nM which did not show any significant CYP suppression or microtubule disruption in hepatocytes.
Conclusions
MMAE was not a CYP inducer in human hepatocytes. However, it caused a concentration-dependent CYP mRNA suppression and activity. The CYP suppression was associated with microtubule disruption, supporting the reports that intact microtubule architecture is required for CYP regulations. The absence of CYP suppression and microtubule disruption in vitro at the clinical plasma concentrations of MMAE (< 10 nM) explains the lack of pharmacokinetic drug interaction between brentuximab vedotin and midazolam, a sensitive CYP3A substrate, reported in patients.


Similar content being viewed by others
References
Tolcher AW. Antibody drug conjugates: lessons from 20 years of clinical experience. Ann Oncol. 2016;27:2168–72.
Reichert JM. Antibodies to watch in 2017. mAbs. 2017;9:167–81.
Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, Larson RA, Erba HP, Stiff PJ, Stuart RK, Walter RB, Tallman MS, Stenke L, Appelbaum FR. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–60.
Van de Donk NWCJ, Dhimolea E. Brentuximab vedotin. mAbs. 2012;4:458–65.
Madan A, Dehaan R, Mudra D, Carroll K, LeCluyse E, Parkinson A. Effect of cryopreservation on cytochrome P-450 enzyme induction in cultured rat hepatocytes. Drug Metab Dispos. 1999;27:327–35.
Schneider CA, Rasband WS, Eliceiri KW. NIH image to imageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Han TH, Gopal AK, Ramchandren R, Goy A, Chen R, Matous JV, Cooper M, Grove LE, Alley SC, Lynch CM, O’Connor OA. CYP3A-mediated drug–drug interaction potential and excretion of Brentuximab Vedotin, an antibody–drug conjugate, in patients with CD30-positive hematologic malignancies J. Clin Pharm. 2013;53:866–77.
Hariprasad N, Ramsden D, Palamanda J, Dekeyser JG, Fahmi OA, Kenny JR, Einolf H, Mohutsky M, Pardon M, Siu AY, Chen L, Sinz M, Jones B, Walsky R, Dallas S, Balani SK, LeCluyse E, Zhang G, Buckley D, and Tweedie D. Considerations from the IQ induction working group in response to drug–drug interaction guidances from regulatory agencies: focus on down-regulation, CYP2C induction and CYP2B6 positive control. Drug Metab Dispos. 2017;45:1049-59.
Dvorak Z, Modriansky M, Pichard-Garcia L, Balaguer P, Vilarem M-J, Ulrichova J, Maurel P, Pascussi J-M. Colchicine down-regulates cytochrome P450 2B6, 2C8, 2C9 and 3A4 in human hepatocytes by affecting their glucocorticoid receptor-mediated regulation. Mol Pharmacol. 2003;64:160–9.
Dvorak Z, Ulrichova J, Modriansky M. Role of microtubules network in CYP genes expression. Curr Drug Metab. 2005;6:545–52.
Acknowledgements
We thank Sekisui XenoTech, LLC for work with CYP induction study, Steve Alley of Seattle Genetics, and Adedigbo Fasanmade of Takeda for the helpful discussions.
Funding
No funding source to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors were compensated employees of either Seattle Genetics, Inc. (THH) or Takeda Pharmaceuticals International Co. (FSW, BM, CQX, WCS, and SKB) when this research was conducted.
Rights and permissions
About this article
Cite this article
Wolenski, F.S., Xia, C.Q., Ma, B. et al. CYP Suppression in Human Hepatocytes by Monomethyl Auristatin E, the Payload in Brentuximab Vedotin (Adcetris®), is Associated with Microtubule Disruption. Eur J Drug Metab Pharmacokinet 43, 347–354 (2018). https://doi.org/10.1007/s13318-017-0455-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-017-0455-5