Abstract
Introduction
This study utilized continuous glucose monitoring data to analyze the effects of switching to treatment with fast-acting insulin aspart (faster aspart) in adults with type 1 diabetes (T1D) in clinical practice.
Methods
A noninterventional database review was conducted in Sweden among adults with T1D using multiple daily injection (MDI) regimens who had switched to treatment with faster aspart as part of basal-bolus treatment. Glycemic data were retrospectively collected during the 26 weeks before switching (baseline) and up to 32 weeks after switching (follow-up) to assess changes in time in glycemic range (TIR; 70–180 mg/dL), mean sensor glucose, glycated hemoglobin (HbA1c) levels, coefficient of variation, time in hyperglycemia (level 1, > 180 to ≤ 250 mg/dL; level 2, > 250 mg/dL), and time in hypoglycemia (level 1, ≥ 54 to < 70 mg/dL; level 2, < 54 mg/dL) (ClinicalTrials.gov Identifier NCT03895515).
Results
Overall, 178 participants were included in the study cohort. The analysis population included 82 individuals (mean age 48.5 years) with adequate glucose sensor data. From baseline to follow-up, statistically significant improvements were reported for TIR (mean increase 3.3%-points [approximately 48 min/day]; p = 0.006) with clinically relevant improvement (≥ 5%) in 43% of participants. Statistically significant improvements from baseline were observed for mean sensor glucose levels, HbA1c levels, and time in hyperglycemia (levels 1 and 2), with no statistically significant changes in time spent in hypoglycemia.
Conclusions
Switching to faster aspart was associated with improvements in glycemic control without increasing hypoglycemia in adults with T1D using MDI in this real-world setting.
Similar content being viewed by others
Why carry out this study? |
Fast-acting insulin aspart (faster aspart) is a modified formulation of insulin aspart with a faster onset of action and a shorter duration of action; however, there is limited real-world evidence available to provide insights into everyday use in adults with type 1 diabetes (T1D) |
This study used continuous glucose monitoring data from clinical practice databases to analyze the potential glycemic consequences of switching to faster aspart among adults with T1D on multiple daily injection regimens |
What was learned from the study? |
Switching to faster aspart was associated with improvements from baseline in glycemic control across various measures without an increased risk of hypoglycemic events |
These results supplement the limited real-world evidence by demonstrating the potential clinical benefits of faster aspart |
Introduction
In individuals with type 1 diabetes (T1D), treatment to achieve effective glycemic control is of central importance because this is associated with delays in the onset of microvascular complications as well as reduced incidence of cardiovascular disease [1, 2]. Insulin treatment strategies aim to maintain glucose levels in the normal physiological range by mimicking the physiological profile of insulin release, which consists of a constant, low-level release and rapid, transient increases in response to food [3, 4]. To achieve this, individuals with T1D can utilize continuous subcutaneous insulin infusion via an insulin pump or a multiple daily injection (MDI) regimen consisting of basal insulin to maintain glucose levels in the preprandial state, and bolus insulin to address physiological insulin needs in response to food intake at mealtimes [5].
Bolus insulin analogues, such as insulin aspart, insulin lispro, and insulin glulisine, have been developed with pharmacokinetic/pharmacodynamic profiles that aim to replicate the rapid mealtime bursts in insulin secretion [6]. Fast-acting insulin aspart (faster aspart) is a modified formulation of insulin aspart with the addition of l-arginine and niacinamide. Compared with insulin aspart, this formulation has a faster onset of action and a shorter duration of action [7, 8].
The phase 3 ONSET 1 and ONSET 8 randomized clinical trials investigated the efficacy and safety of faster aspart in individuals with T1D using MDI regimens. In ONSET 1, participants were randomized to faster aspart or insulin aspart, both in combination with insulin detemir. Treatment with mealtime (preprandial) or post-meal faster aspart was associated with small but significantly greater improvements in glycated hemoglobin (HbA1c) levels from baseline compared with mealtime insulin aspart [9, 10]. Moreover, compared with insulin aspart, mealtime faster aspart was associated with lower 2-h postprandial plasma glucose (PPG) values in response to a standardized meal test. Overall, the rates of blood glucose-confirmed hypoglycemic episodes (plasma glucose value < 56 mg/dL) or severe hypoglycemic episodes (events requiring assistance of another person) were similar for faster aspart and insulin aspart; however, during the first hour after the start of a meal, higher rates were observed in the mealtime faster aspart arm than with insulin aspart. In the ONSET 8 trial, individuals with T1D were randomized to receive faster aspart or insulin aspart, both in combination with insulin degludec; in this trial, noninferiority to mealtime insulin aspart was confirmed for mealtime or post-meal faster aspart for change from baseline in HbA1c levels. Furthermore, for mealtime faster aspart versus insulin aspart, superiority was demonstrated in the 1-h PPG increment after a meal. The overall rates of blood glucose-confirmed hypoglycemia or severe hypoglycemia (events requiring assistance of another person) were similar between treatments arms with statistically significantly lower rates observed 3–4 h after a meal in the faster aspart arm compared with insulin aspart [11].
Although HbA1c levels are considered to be the gold standard for measuring diabetes outcomes, clinically meaningful outcomes beyond HbA1c are recommended in the investigation of T1D therapies [12]. Data derived from continuous glucose monitoring (CGM) can supplement HbA1c by providing a more comprehensive display of glucose control [13]. As such, additional investigations with CGM may provide further insights into the effects of treatment on daily glucose variations. Previous observational studies conducted in Germany (GoBolus) [14] and Belgium [15] have reported results in individuals using CGM (real-time CGM [rtCGM] or intermittently scanned CGM [isCGM]). In the GoBolus study, switching to faster aspart was associated with improvements in estimated HbA1c, increased time in range (TIR [70–180 mg/dL]), and decreased time in hyperglycemia (level 1 [> 180 mg/dL] and level 2 [> 250 mg/dL]) compared with baseline; however, no change in time in hypoglycemia (< 54 mg/dL) was observed [14]. In the Belgian study, switching to faster aspart was associated with increased TIR (70–180 mg/dL), less time in hyperglycemia (level 1 [> 180 mg/dL] and level 2 [> 250 mg/dL]), and less time in level 2 hypoglycemia (< 54 mg/dL), but no significant improvement in HbA1c from baseline levels [15].
To our knowledge, only the two studies described above have previously investigated the effectiveness of faster aspart in individuals with T1D receiving MDI therapy by utilizing CGM data [14, 15]. It is important to confirm the effects of new medications in different regions/countries because standard of care and management practices may differ from one region/country to another. As such, further real-world studies are beneficial to confirm the effects of these medications under different circumstances. The objective of this study was to expand on the real-world evidence available by utilizing CGM data from clinical practice databases to analyze potential glycemic consequences of switching to faster aspart among adults with T1D on MDI regimens.
Methods
Study Design and Participants
This was a noninterventional chart and database review study conducted in seven diabetes clinics in Sweden (ClinicalTrials.gov Identifier NCT03895515). Data were retrospectively collected from electronic medical records, and CGM (rtCGM or isCGM, referred to as “CGM” hereafter) records. This study was conducted in accordance with the Declaration of Helsinki (2004) and the Guidelines for Good Pharmacoepidemiology Practices (2011) [16, 17]. The study was approved by the Swedish Ethical Review Authority (Uppsala; ID number 2019-01084) and written informed consent was obtained before any study-related activities.
Study eligibility criteria were adults (aged ≥ 18 years) with diagnosed T1D at least 12 months before initiation of faster aspart treatment; switched to a basal-bolus MDI regimen with faster aspart from a basal-bolus regimen with any other bolus insulin; previously treated with basal-bolus insulin throughout the 26 weeks before switching to faster aspart; had no changes to basal insulin use in the 26 weeks before switching to faster aspart or the 26 weeks after switching; and used CGM in the 26 weeks before faster aspart initiation and in the 26 weeks after initiation, with no change to the CGM device after switching to faster aspart. All participants had received their first prescription of faster aspart in the period from September 1, 2017 to April 30, 2019. Key exclusion criteria were previous use of faster aspart; use of an insulin pump (continuous subcutaneous insulin infusion) in the 26 weeks before or after faster aspart initiation; and use of noninsulin glucose-lowering drugs in addition to insulin treatments in the 26 weeks before or after faster aspart initiation. All participants had received standard clinical care; i.e., no standardized insulin dosing algorithms had been used, and they had been encouraged to self-manage their glucose control using real-time and time trend CGM data.
The index date was defined as the date of the first faster aspart prescription. The study timeline included a pre-index period, defined as 182 days (26 weeks) up to and including the index date, and a post-index period after the index date (Fig. 1). Baseline levels of glucose were estimated on the basis of the CGM data available during the last 28 days of the pre-index period, whereas baseline HbA1c was based on the measurement closest to the index date during the last 84 days of the pre-index period. The 26-week follow-up assessed data collected during the post-index period – sensor glucose measurements were analyzed from day 141 (start of week 21) to day 224 (end of week 32), and HbA1c measurements were assessed from day 85 (start of week 13) to day 224 (end of week 32), according to data availability and by considering the measurements closest to day 182 (end of week 26) per patient (Fig. 1).
Study timeline. The index date was defined as the date of the first faster aspart prescription. The pre-index period was defined as 182 days (26 weeks) up to and including the index date, and the post-index period was defined as 182–224 days (26–32 weeks) after the index date. Sensor glucose measurements were analyzed from day 141 (start of week 21) to day 224 (end of week 32). HbA1c measurements were analyzed from day 85 (start of week 13) to day 224 (end of week 32), according to data availability and by considering the measurement closest to day 182 (end of week 26) per patient. For the analysis, only one 2-week period with sufficient glucose data quality (≥ 70% of available CGM measurements) was included per participant for each of the pre-index baseline and post-index follow-up periods. Faster aspart fast-acting insulin aspart, HbA1c glycated hemoglobin
Data Collection
CGM data were utilized for glucose monitoring. All participants utilized a CGM device with a serial number and/or username associated with a Diasend account (Glooko Diasend; Glooko Inc.). Glooko Inc. (Mountain View, CA, USA) extracted monitoring data from Diasend. Data collected from electronic medical records and readings from Diasend were entered into a database for statistical analysis. For these analyses, only one 2-week period with sufficient glucose data quality (≥ 70% of available CGM measurements) was included per participant for each of the pre-index and post-index periods. Two-week periods with less than 70% of available measurements were not considered in the analyses. Individuals without a 2-week period fulfilling the criteria for either pre-index or post-index periods were excluded from the analysis.
Two analysis sets were defined: the primary analysis set included all eligible individuals with at least 26 weeks of continuous exposure to faster aspart after the index date; the sensor glucose analysis set consisted of all individuals in the primary analysis set who met the requirement of sufficient glucose sensor data quality during baseline and follow-up.
Outcomes
The primary endpoint was the change in percentage units of TIR (70–180 mg/dL [3.9–10.0 mmol/L]) from initiation of faster aspart to post-index follow-up. Secondary endpoints for change from baseline were also analyzed for mean sensor glucose; glycemic variability (measured with coefficient of variation [%CV]); percentage of time spent in level 1 hyperglycemia (> 180 mg/dL [10.0 mmol/L] to ≤ 250 mg/dL [13.9 mmol/L]); percentage of time spent in level 2 hyperglycemia (> 250 mg/dL [13.9 mmol/L]); percentage of time spent in level 1 hypoglycemia (≥ 54.0 mg/dL [3.0 mmol/L]) to < 70 mg/dL [3.9 mmol/L]); percentage of time spent in level 2 hypoglycemia (< 54 mg/dL [3.0 mmol/L]); and HbA1c levels.
Statistical Analysis
A target sample size of 250 participants was calculated to detect a minimum acceptable clinically significant change in TIR of 2% with a standard deviation of 2.59 h per day (10.79%), based on a pilot study from Sweden. The dropout risk was expected to be 15%. On the basis of these assumptions, a sample size of 250 would have sufficient power of 80% to detect the primary endpoint. The analyses of the primary and secondary endpoints were conducted in the sensor glucose analysis set. Changes in primary and secondary endpoints from baseline to post-index follow-up were tested using t tests with 5% significance level for p values. Linear regression models were used to measure change from baseline; in the fully adjusted model, change in the variable of interest was the dependent variable, adjusted for the independent variables sex, age at index, diabetes duration, and the baseline value of the variable of interest. The assumption that linear regression model residuals are normally distributed was assessed using Q-Q plots of the residuals. When appropriate, a log transformation was applied, or an appropriate nonparametric approach was adopted.
Results
Participant Characteristics
Overall, 178 individuals with T1D were included in the primary analysis set. Among these participants, 167 had data available in the Glooko database, but the completeness and timing of sensor glucose recordings were variable (Supplementary Material Fig. S1), and only 82 had sufficient data in the period before and after faster aspart initiation for inclusion in the sensor glucose analysis set (Fig. 2). Baseline participant characteristics were similar between these cohorts. In the sensor glucose analysis set, 50% of participants were male and the mean age was 48.5 years, ranging from 19.0 years to 84.3 years. Mean diabetes duration was 25.7 years, ranging from 2.0 years to 74.0 years (Table 1).
Participant recruitment in the study. A 2-week period with sufficient glucose data quality (≥ 70% of available sensor glucose measurements) was required per participant for each of the pre-index baseline and post-index follow-up periods. Faster aspart fast-acting insulin aspart, isCGM intermittently scanned continuous glucose monitoring, rtCGM real-time continuous glucose monitoring
Primary Endpoint: TIR in the Sensor Glucose Analysis Set
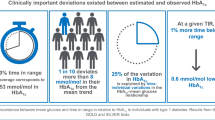
Mean (standard deviation [SD]) TIR (70–180 mg/dL) was 55.3%-points (16.2%-points) at baseline and 58.7%-points (15.8%-points) at post-index follow-up (Fig. 3A), corresponding with a mean increase in TIR of 3.3%-points (95% confidence interval (CI) 0.99%-points to 5.6%-points) from baseline to follow-up, or approximately 48 min per day; this change was statistically significant (p = 0.006) (Fig. 3B). An increase in TIR of at least 2% was registered in 46 participants (56%), and of at least 5% in 35 participants (43%) from baseline to follow-up.
In linear regression analyses, a lower baseline TIR was associated with larger increases in TIR during follow-up (adjusted model coefficient: − 0.267; p = 0.0002; unadjusted model coefficient: − 0.239; p = 0.0008) (Supplementary Material Fig. S2). Among participants with over 70% TIR at baseline, the mean (SD) TIR at follow-up changed by − 1.4%-points (9.8%-points) compared with an increase of 4.8%-points (10.5%-points) among those with 70% or less TIR at baseline.
Secondary Endpoints in the Sensor Glucose Analysis Set
Statistically significant changes in mean sensor glucose levels were observed from baseline to follow-up. The mean change from baseline was − 0.3 (95% CI − 0.58 to − 0.08) mmol/L (p = 0.012).
The mean time spent in level 1 hyperglycemia was significantly lower at follow-up than at baseline (Fig. 3a). Mean change from baseline was statistically significant in a t test, − 3.2%-points (95% CI − 5.7%-points to − 0.6%-points; p = 0.017), equivalent to approximately 45 min per day (Fig. 3b). Moreover, the mean time spent in level 2 hyperglycemia also reduced from baseline to follow-up (Fig. 3a). The mean change from baseline was statistically significant: − 2.6%-points (95% CI − 4.3%-points to − 0.9%-points; p = 0.003), equivalent to approximately 38 min per day (Fig. 3b).
No statistically significant changes were observed for percentage of time spent in level 1 or level 2 hypoglycemia; patients spent approximately 7% of their time in level 1 hypoglycemia and approximately 2% in level 2 hypoglycemia at both baseline and follow-up (Fig. 3a, b).
At follow-up, the %CV in glucose measurements provided by glucose sensors reduced from baseline by 0.6%-points; however, this change was not statistically significant (p = 0.331).
Statistically significant reductions in HbA1c levels were reported from baseline to follow-up. The mean change from baseline was − 2.9 (95% CI − 5.2 to − 0.6) mmol/mol (p = 0.015). A higher baseline HbA1c level (mmol/mol) was associated with a greater reduction in HbA1c at follow-up, as shown in linear regression analyses using both adjusted (coefficient: − 0.358; p = 0.0002) and unadjusted (coefficient: − 0.337; p = 0.0001) models with change in HbA1c as the dependent variable.
Discussion
In this multicenter study analyzing data from real-world clinical practice in Sweden, we found that switching to faster aspart was associated with improved glycemic control in T1D. There were statistically significant improvements from baseline in both TIR and exposure to level 1 and 2 hyperglycemia. Moreover, no significant changes from baseline in time spent in level 1 and 2 hypoglycemia were registered, providing evidence that switch to faster aspart did not lead to any undesirable increased risk of hypoglycemia over the course of the day. The observed reductions in mean sensor glucose and HbA1c levels following the introduction of faster aspart treatment also further provide evidence for improved glycemic control following switching to insulin faster aspart in real-world clinical practice.
In this study, CGM data were recorded, allowing for a detailed analysis of the glycemic changes across participants. Collection of glycemic data in addition to HbA1c provides a more complete description of overall glycemic control, including time with hypoglycemia, hyperglycemia, and glucose fluctuations. Moreover, various ranges of mean glucose profiles may be associated with a specific HbA1c level [18]. As such, data from CGM recordings may optimize our understanding of the effectiveness of insulin treatment by providing a clear picture of glucose excursions and other glucose patterns [19]. Furthermore, CGM allows for detailed monitoring of glycemic variability, which some studies suggest may have associations with long-term complications such as retinopathy and neuropathy [20, 21].
Because faster aspart is administered at mealtimes, an important target is to stabilize PPG levels. The observed increase from baseline in TIR (approximately 48 min per day) was similar to the decrease in time spent in level 1 hyperglycemia (approximately 45 min per day) and level 2 hyperglycemia (approximately 38 min per day), a result that is consistent with the PPG improvements that were reported in patients with T1D following a switch to faster aspart in the ONSET 1 and ONSET 8 MDI trials [9,10,11].
The potential benefits of switching to faster aspart were demonstrated in the overall study population (as shown by the statistically significant 3.3%-point increase in TIR from baseline and of at least 5% in 43% of participants, the latter being considered as a clinically significant improvement according to current guidelines [19]). Specifically, participants with 70% or less TIR at baseline had a larger increase in TIR during the study than those with a baseline TIR over 70%. It is worth noting that approximately 40% of the study population had a TIR less than 50% at baseline and they may benefit from a stricter glucose management program.
There are limited data available from previous studies to directly compare TIR in the study population with that in a nationwide T1D population in Sweden and to gauge the generalizability of our findings. However, based on data from the Swedish National Diabetes Register, the mean HbA1c level for adult individuals with T1D in specialist clinics nationwide was 59.9 mmol/mol. The baseline mean HbA1c levels reported in this study were slightly lower (56.5 mmol/mol in the primary analysis set and 56.1 mmol/mol in the sensor glucose analysis set), suggesting that levels of glycemic control may be reasonably representative of the population [22].
The main challenge for this study was the lack of availability of patient data from the Glooko database that met the criteria for CGM analysis (Supplementary Material Fig. S1). For inclusion in the analysis, CGM data had to be available for a 2-week period with 70% or more CGM coverage; however, many participants did not have adequate glucose data collection. This finding suggests that although CGM can potentially provide a wealth of data for understanding glycemic control, real-world studies can be limited by inconsistent data uploads by participants. This is also in alignment with previous research that has reported substantial nonadherence to CGM in clinical practice, and observational studies in which limited availability of CGM data resulted in limited sample sizes for analyses [14, 23]. As such, future real-world studies may benefit from providing increased support to CGM users as part of clinical practice, such as encouragement to perform regular data uploads or use of other technology such as mobile applications. Moreover, refinements in the collection of CGM data, such as integration of data into electronic health records in clinical practice, may be beneficial for future research [24].
The efficacy and safety of faster aspart was previously demonstrated in the ONSET clinical trial program; however, there is a need for real-world studies to complement the evidence available by providing insights into everyday use in adults with T1D. This is the first real-world analysis to report the effects of faster aspart in Sweden and these results are generally consistent with real-world studies conducted in individuals treated with faster aspart in other countries. In the GoBolus study, treatment with faster aspart as part of an MDI regimen was also associated with improvements from baseline for TIR, time in hyperglycemia, and HbA1c levels [14]. The improvement in TIR from baseline to 24-week follow-up was equivalent to approximately 46 min per day, which is similar to the 48 min per day reported in this study. In a real-world study in Belgium that investigated switching to faster aspart among individuals using MDI (93.4% of participants) or continuous subcutaneous insulin infusion (6.6% of participants), improvements from baseline were observed in TIR, time in hyperglycemia, and time in hypoglycemia. In the 6 months after switching to faster aspart, the TIR reportedly increased from baseline by approximately 57 min per day; this difference was even larger at 12 months, when the observed increase from baseline TIR was 75 min per day. Despite this finding, no significant change in HbA1c level was reported [15]. At the 6-month follow-up, the proportion of individuals with improvements from baseline TIR of 5% or more was approximately 40%, similar to the 43% reported in our study [15]. In concert with our findings, following treatment with faster aspart, glucose variability (%CV) was not lowered from baseline in the GoBolus study, whereas it was slightly lowered, albeit statistically significantly, in the Belgian trial [14, 15]. Given that %CV represents the magnitude of high and low glucose excursions, this may imply that reductions in both time in hyper- and hypoglycemia are needed to influence this measure.
Unlike the previous GoBolus and Belgian studies, the present study further demonstrated that baseline glycemic measures (including TIR and HbA1c) have significant associations with the degree of change in glycemic control after switching to faster aspart. As such, although eligibility criteria in this study were similar to previous observational studies, results cannot be directly compared owing to differences in the participant populations. When considering baseline characteristics across studies, the mean baseline TIR was slightly higher in this study (55.3%) than in GoBolus (46.9%) [14] and the Belgian study (50.3%) [15]. In addition, the mean HbA1c level at baseline varied across studies: 56.1 mmol/mol in this study compared with 64.8 mmol/L in GoBolus [14] and 62.2 mmol/mol in the Belgian study [15]. These measures suggest that baseline glycemic control was better in this study compared with GoBolus and the Belgian study; however, despite this, similar improvements in glycemic control were observed across studies following initiation of faster aspart, supporting the generalizability of these results.
Strengths of this study included the multicenter design, making data more representative, and the collection of real-world data, which allowed for elucidation of the effects of faster aspart in individuals with T1D in their day-to-day life. Furthermore, study inclusion criteria ensured that participants used the same type of basal insulin and rtCGM or isCGM system during follow-up. There were also several limitations associated with this study. First, the follow-up time was relatively short (up to 32 weeks) and future studies covering a longer time period may be required to clarify the current observations. Owing to difficulties in recruiting, the study did not achieve the planned sample size of 250 participants and a substantial proportion of participants did not fit the criteria for inclusion in the glucose analysis set because they did not have adequate glucose data; that is, they did not frequently scan and upload their glucose measurements. Nonetheless, the results showed a statistically significant 3.3%-point increase in TIR from baseline following switching to faster aspart. In addition, baseline clinical characteristics were almost identical in the final glucose sensor analysis set compared to the full primary analysis set, which suggests that there was no selection bias in the former group. Moreover, the participant population was of similar size to the CGM analysis population in the GoBolus study, which also reported statistically significant results; the lack of complete CGM data was also a limiting factor in GoBolus (isCGM data available in 92 of 243 participants) [14]. It is often challenging to retrieve a representative control group in real-life studies of this type and, therefore, control groups have also been lacking in previous similar evaluations [14, 15]. Insulin injection timing and dosing data were also not collected as part of this work; collecting this information in future studies could provide further context to the observed changes in glycemic control. In addition, participant satisfaction was not assessed, but collection of such data in future studies could provide a more complete understanding of patient experiences following switching to treatment with faster aspart. Finally, residual confounding cannot be excluded owing to the observational nature of the study.
Conclusion
Switching to faster aspart was associated with improvements from baseline in glycemic control in individuals with T1D across various measures, with 43% of participants experiencing a clinically significant improvement (≥ 5%) in TIR from baseline. Furthermore, the study adds to the body of evidence regarding the safety of faster aspart in clinical practice because there was no apparent increased risk of hypoglycemic events after switching to faster aspart. This study provides further evidence of potential clinical benefits of faster aspart treatment in the real world.
References
Diabetes, Control Complications Trial/Epidemiology of Diabetes, Interventions Complications Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39(5):686–93.
Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Home PD. Plasma insulin profiles after subcutaneous injection: how close can we get to physiology in people with diabetes? Diabetes Obes Metab. 2015;17(11):1011–20.
Kruszynska YT, Home PD, Hanning I, Alberti KG. Basal and 24-h C-peptide and insulin secretion rate in normal man. Diabetologia. 1987;30(1):16–21.
Holt RIG, De Vries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021;64(12):2609–52.
Home PD. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes Metab. 2012;14(9):780–8.
Kildegaard J, Buckley ST, Nielsen RH, et al. Elucidating the mechanism of absorption of fast-acting insulin aspart: the role of niacinamide. Pharm Res. 2019;36(3):49.
Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56(5):551–9.
Russell-Jones D, Bode BW, De Block C, et al. Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (Onset 1). Diabetes Care. 2017;40(7):943–50.
Mathieu C, Bode BW, Franek E, et al. Efficacy and safety of fast-acting insulin aspart in comparison with insulin aspart in type 1 diabetes (Onset 1): a 52-week, randomized, treat-to-target, phase III trial. Diabetes Obes Metab. 2018;20(5):1148–55.
Buse JB, Carlson AL, Komatsu M, et al. Fast-acting insulin aspart versus insulin aspart in the setting of insulin degludec-treated type 1 diabetes: efficacy and safety from a randomized double-blind trial. Diabetes Obes Metab. 2018;20(12):2885–93.
Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA(1c) for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. helmsley charitable trust, the pediatric endocrine society, and the T1D exchange. Diabetes Care. 2017;40(12):1622–30.
Beyond A1C Writing Group. Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care. 2018;41(6):e92–4.
Danne T, Axel Schweitzer M, Keuthage W, et al. Impact of fast-acting insulin aspart on glycemic control in patients with type 1 diabetes using intermittent-scanning continuous glucose monitoring within a real-world setting: the GoBolus study. Diabetes Technol Ther. 2021;23(3):203–12.
Billion L, Charleer S, Verbraeken LJR, et al. Glucose control using fast-acting insulin aspart in a real-world setting: a 1-year, two-centre study in people with type 1 diabetes using continuous glucose monitoring. Diabetes Obes Metab. 2021;23(12):2716–27.
World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects 52nd WMA General Assembly, Edinburgh, Scotland, October 2000 Last amended with Note of Clarification on Paragraph 29 by the WMA General Assembly, Washington 2002, and Note of Clarification on Paragraph 30 by the WMA General assembly, Tokyo 2004. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed Jan 2023.
International Society for Pharmacoepidemiology (ISPE). Guidelines for Good Pharmacoepidemiology Practices (GPP). https://www.pharmacoepi.org/resources/policies/guidelines-08027/. Accessed Jan 2023.
Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA(1c) alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–9.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603.
Sartore G, Chilelli NC, Burlina S, Lapolla A. Association between glucose variability as assessed by continuous glucose monitoring (CGM) and diabetic retinopathy in type 1 and type 2 diabetes. Acta Diabetol. 2013;50(3):437–42.
Šoupal J, Škrha J Jr, Fajmon M, et al. Glycemic variability is higher in type 1 diabetes patients with microvascular complications irrespective of glycemic control. Diabetes Technol Ther. 2014;16(4):198–203.
Swedish National Diabetes Register. Nationwide results 1996–2020. 2020. https://www.ndr.nu/pdfs/NationWideResults_1996-2020.pdf. Accessed May 11 2022.
Yu S, Varughese B, Li Z, Kushner PR. Healthcare resource waste associated with patient nonadherence and early discontinuation of traditional continuous glucose monitoring in real-world settings: a multicountry analysis. Diabetes Technol Ther. 2018;20(6):420–7.
Espinoza J, Xu NY, Nguyen KT, Klonoff DC. The need for data standards and implementation policies to integrate CGM data into the electronic health record. J Diabetes Sci Technol. 2021. https://doi.org/10.1177/19322968211058148.
Acknowledgements
Authors would like to thank Lars Berg for his support and contributions to this study, and Sofia Salö, Novo Nordisk for review and input to the manuscript.
Funding
This study and the journal Rapid Service Fee were funded by Novo Nordisk A/S. Medical writing support was funded by Novo Nordisk A/S.
Medical Writing/Editorial Assistance
Medical writing support was provided by Kate Ward from Oxford PharmaGenesis, Oxford, UK, funded by Novo Nordisk A/S.
Author Contributions
Marcus Lind, Magnus Löndahl and Jan Bolinder contributed to the design of the study. Tariq Halasa performed data analysis. All authors contributed to the acquisition, analysis, or interpretation of study data, and participated in preparing the manuscript, with the support of medical writing services. All authors agreed with the results and conclusions of the study and approved the submitted version of the manuscript.
Disclosures
Marcus Lind has received honoraria or has been a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk, and received research grants from Eli Lilly and Novo Nordisk. Sergiu Bogdan Catrina has no conflicts of interest to disclose. Neda Rajamand Ekberg has, on behalf of her employer, received gratuities for lecturing and participating in scientific boards for Novo Nordisk. Sofia Gerward and Tariq Halasa are employees of Novo Nordisk A/S. Jarl Hellman has served on advisory boards or lectured for Abbot, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, MSD, Nordic Infucare, Novo Nordisk, Rubin Medical, and Sanofi. Detlef Hess has stock or stock options for AstraZeneca. Magnus Löndahl has received honoraria for consulting and/or lecture fees and/or unrestricted research grants from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, and Sanofi. Veronica Qvist has no conflicts of interest to disclose. Jan Bolinder has received honoraria for consulting and/or lecture fees from Abbott Diabetes Care, MannKind Corporation, Nanexa, Nordic InfuCare, Novo Nordisk, and Sanofi.
Compliance with Ethics Guidelines
This study was conducted in accordance with the Declaration of Helsinki (2004) and the Guidelines for Good Pharmacoepidemiology Practices (2011). The project was approved by the Swedish Ethical Review Authority (Uppsala; ID number 2019-01084) which covered all seven diabetes clinics included in the study. Written informed consent to participate was obtained before any study-related activities; the informed consent form provided individuals with information regarding the purpose, design and setting of the study, the potential risks and/or benefits of participation, and sharing of personal data registered in Diasend and electronic medical records.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lind, M., Catrina, SB., Ekberg, N.R. et al. Fast-Acting Insulin Aspart in Patients with Type 1 Diabetes in Real-World Clinical Practice: A Noninterventional, Retrospective Chart and Database Study. Diabetes Ther 14, 1563–1575 (2023). https://doi.org/10.1007/s13300-023-01444-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01444-y