Abstract
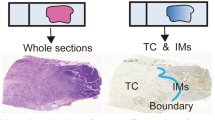
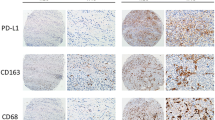
Macrophages play a critical role in the initiation and progression of various solid tumors. However, their prognostic significance in pancreatic ductal adenocarcinoma (PDAC) is poorly understood. This study investigated the distribution patterns of macrophages in PDAC and possible association with the overall survival (OS). We found significant differences in macrophage density (identified by CD68 and CD163 immunopositivity; p < 0.001 for both) between primary cancer and paired adjacent normal tissues. Most macrophages in cancerous pancreatic tissues were located in the stroma rather than the islets (p = 0.032 and p < 0.001). We also demonstrated that a high total macrophage density (characterized by CD68 immunopositivity) correlated with an absence of jaundice before surgery (p = 0.03) and that a high density of M2 macrophages (characterized by CD163 immunopositivity) in the stroma strongly correlated with the tumors located in the tail and body of the pancreas (p = 0.04). In addition, OS was shorter in patients with high-density M2 macrophage infiltration than in those with low-density M2 macrophage infiltration (p = 0.012). Moreover, multivariate analysis revealed that dense M2 macrophage infiltration into the stroma was an independent prognostic factor for PDAC patients (p = 0.02).



Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–8.
Liu Y, Du L. Role of pancreatic stellate cells and periostin in pancreatic cancer progression. Tumor Biol. 2015;36:3171–7.
Tang D, Gao J, Wang S, Yuan Z, Ye N, Chong Y, et al. Apoptosis and anergy of t cell induced by pancreatic stellate cells-derived galectin-1 in pancreatic cancer. Tumor Biol. 2015;36:5617–26.
Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–72.
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–55.
van Furth R. Origin and turnover of monocytes and macrophages; cell kinetics of the inflammatory reaction. Springer; 1989. p. 125–50.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96.
Yoshikawa K, Mitsunaga S, Kinoshita T, Konishi M, Takahashi S, Gotohda N, et al. Impact of tumor‐associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci. 2012;103:2012–20.
Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–9.
An T, Sood U, Pietruk T, Cummings G, Hashimoto K, Crissman J. In situ quantitation of inflammatory mononuclear cells in ductal infiltrating breast carcinoma. Relation to prognostic parameters. Am J Pathol. 1987;128:52.
Hiraoka K, Zenmyo M, Watari K, Iguchi H, Fotovati A, Kimura YN, et al. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Cancer Sci. 2008;99:1595–602.
Lin EY, Li J-F, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46.
Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6.
Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol. 2013;4:210.
Chen SJ, Zhang QB, Zeng LJ, Lian GD, Li JJ, Qian CC, et al. Distribution and clinical significance of tumour-associated macrophages in pancreatic ductal adenocarcinoma: a retrospective analysis in china. Curr Oncol. 2015;22:e11–9.
Jiao F, Hu H, Han T, Yuan C, Wang L, Jin Z, et al. Long noncoding RNA MALAT-1 enhances stem cell-like phenotypes in pancreatic cancer cells. Int J Mol Sci. 2015;16:6677–93.
Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306.
Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376.
De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–86.
Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the m2-like macrophage population. Cancer Res. 2014;74:24–30.
Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–26.
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–23.
Shabo I, Svanvik J. Expression of macrophage antigens by tumor cells; cell fusion in health and disease. Springer; 2011. p. 141–50.
Maniecki MB, Møller HJ, Moestrup SK, Møller BK. CD163 positive subsets of blood dendritic cells: the scavenging macrophage receptors CD163 and CD91 are coexpressed on human dendritic cells and monocytes. Immunobiology. 2006;211:407–17.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81171887, 91229117, 81502017, 81502018, and 81572315), by Research Grant from Shanghai Hospital Development Center (SHDC12014128), by Songjiang Liandong Program (0702N14002), and in part by the National Key Clinical Discipline Oncology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Written informed consent and approval from the Ethics Committees of Shanghai General Hospital were obtained for the use of these clinical materials for research purposes.
Conflicts of interest
None
Additional information
Hai Hu, Jun-Jie Hang and Ting Han contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hu, H., Hang, JJ., Han, T. et al. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumor Biol. 37, 8657–8664 (2016). https://doi.org/10.1007/s13277-015-4741-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4741-z