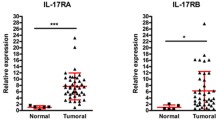
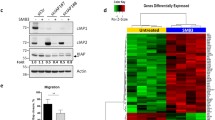
Abstract
Interleukin-8 (IL-8) serves as a prognostic marker for breast cancer, and its expression level correlates with metastatic breast cancer and poor prognosis. Here, we investigated the levels of IL-8 expression in a variety of breast cancer cells and the regulatory mechanism of IL-8 in triple-negative breast cancer (TNBC) cells. Our results showed that IL-8 expression correlated positively with overall survival in basal-type breast cancer patients. The levels of IL-8 mRNA expression and protein secretion were significantly increased in TNBC cells compared with non-TNBC cells. In addition, the invasiveness of the TNBC cells was dramatically increased by IL-8 treatment and then augmented invasion-related proteins such as matrix metalloproteinase (MMP)-2 or MMP-9. We observed that elevated IL-8 mRNA expression and protein secretion were suppressed by a specific MEK1/2 inhibitor, UO126. In contrast, the overexpression of constitutively active MEK significantly increased the level of IL-8 mRNA expression in BT474 non-TNBC cells. Finally, we investigated the effect of UO126 on the tumorigenecity of TNBC cells. Our results showed that anchorage-independent growth, cell invasion, and cell migration were also decreased by UO126 in TNBC cells. As such, we demonstrated that IL-8 expression is regulated through MEK/ERK-dependent pathways in TNBC cells. A diversity of MEK blockers, including UO126, may be promising for treating TNBC patients.





Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi:10.1001/jama.295.21.2492.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi:10.1158/1078-0432.CCR-06-3045.
Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010;23:123–33. doi:10.1038/modpathol.2009.145.
Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer Lett. 2008;113:2638–45. doi:10.1002/cncr.23930.
Otvos Jr L, Surmacz E. Targeting the leptin receptor: a potential new mode of treatment for breast cancer. Expert Rev Anticancer Ther. 2011;11:1147–50. doi:10.1586/era.11.109.
Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi:10.1158/1078-0432.CCR-07-4843.
Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer. 1997;72:937–41.
Ivarsson K, Ekerydh A, Fyhr IM, Janson PO, Brannstrom M. Upregulation of interleukin-8 and polarized epithelial expression of interleukin-8 receptor A in ovarian carcinomas. Acta Obstet Gynecol Scand. 2000;79:777–84.
Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–7.
Miller LJ, Kurtzman SH, Wang Y, Anderson KH, Lindquist RR, Kreutzer DL. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res. 1998;18:77–81.
Baud V, Liu ZG, Bennett B, Suzuki N, Xia Y, Karin M. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–308.
Lee LF, Li G, Templeton DJ, Ting JP. Paclitaxel (Taxol)-induced gene expression and cell death are both mediated by the activation of c-Jun NH2-terminal kinase (JNK/SAPK). J Biol Chem. 1998;273:28253–60.
Scherle PA, Jones EA, Favata MF, Daulerio AJ, Covington MB, Nurnberg SA, et al. Inhibition of map kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J Immunol. 1998;161:5681–6.
Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, et al. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine+. 2005;29:275–82. doi:10.1016/j.cyto.2004.11.005.
Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Konieczny D, et al. Elevation of circulating interleukin-8 is related to lymph node and distant metastases in esophageal squamous cell carcinomas—implication for clinical evaluation of cancer patient. Cytokine+. 2008;41:232–9. doi:10.1016/j.cyto.2007.11.011.
Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013;73:3470–80. doi:10.1158/0008-5472.CAN-12-4524-T.
Wang Y, Xu RC, Zhang XL, Niu XL, Qu Y, Li LZ, et al. Interleukin-8 secretion by ovarian cancer cells increases anchorage-independent growth, proliferation, angiogenic potential, adhesion and invasion. Cytokine+. 2012;59:145–55. doi:10.1016/j.cyto.2012.04.013.
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–31. doi:10.1007/s10549-009-0674-9.
Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–62. doi:10.1158/1078-0432.CCR-04-0812.
Derin D, Soydinc HO, Guney N, Tas F, Camlica H, Duranyildiz D, et al. Serum IL-8 and IL-12 levels in breast cancer. Med Oncol. 2007;24:163–8.
Chen Y, Chen L, Li JY, Mukaida N, Wang Q, Yang C, et al. ERbeta and PEA3 co-activate IL-8 expression and promote the invasion of breast cancer cells. Cancer Biol Ther. 2011;11:497–511.
Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, et al. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004;109:507–15. doi:10.1002/ijc.11724.
Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 1825;2012:29–36. doi:10.1016/j.bbcan.2011.10.001.
Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, et al. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6:2104–19.
Kim S, Lee J, Jeon M, Nam SJ, Lee JE. Elevated TGF-beta1 and -beta2 expression accelerates the epithelial to mesenchymal transition in triple-negative breast cancer cells. Cytokine+. 2015;75:151–8. doi:10.1016/j.cyto.2015.05.020.
Natarajan R, Gupta S, Fisher BJ, Ghosh S, Fowler 3rd AA. Nitric oxide suppresses IL-8 transcription by inhibiting c-Jun N-terminal kinase-induced AP-1 activation. Exp Cell Res. 2001;266:203–12. doi:10.1006/excr.2001.5218.
Wu GD, Lai EJ, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J Biol Chem. 1997;272:2396–403.
Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–6.
Suzuki M, Tetsuka T, Yoshida S, Watanabe N, Kobayashi M, Matsui N, et al. The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-alpha- or IL-1beta-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 2000;465:23–7.
Penson RT, Kronish K, Duan Z, Feller AJ, Stark P, Cook SE, et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10:33–41.
Bhola NE, Balko JM, Dugger TC, Kuba MG, Sanchez V, Sanders M, et al. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–58. doi:10.1172/JCI65416.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3418), and by a Samsung Biomedical Research Institute grant (SMX1131701).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement 1
The co-relation between IL-8 expression and relapse-free survival in luminal B- and HER2-type breast cancer patients. We analyzed the clinical value of IL-8 in luminal B- and HER2-type breast cancer patients using a public database [Kaplan–Meier plotter database (http://kmplot.com/breast)]. (A) Relapse-free survival of luminal B type breast cancer patients. (B) Relapse-free survival of HER2 type breast cancer patients. Lum: Luminal. (GIF 10 kb)
Supplement 2
IL-8-induced cell invasion is suppressed by SB225002 in MDA-MB231 cells. (A) We analyzed the levels of MEK and ERK phosphorylation using whole cell lysates in BT474 and MDA-MB231 cells. The levels of phospho-MEK, phospho-ERK, and β-actin expression were analyzed by western blotting. (B) MDA-MB231 cells was pretreated with 10 μM SB225002 for 30 min and then treated with 20 ng/ml IL-8 for 16 h. The cells on the bottom side of the filter were fixed and stained. Cell morphology was analyzed using a CK40 inverted microscope. These results are representative of three independent experiments. Con: control, SB: SB225002. (GIF 14 kb)
Rights and permissions
About this article
Cite this article
Kim, S., Lee, J., Jeon, M. et al. MEK-dependent IL-8 induction regulates the invasiveness of triple-negative breast cancer cells. Tumor Biol. 37, 4991–4999 (2016). https://doi.org/10.1007/s13277-015-4345-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4345-7