Abstract
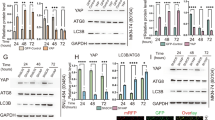
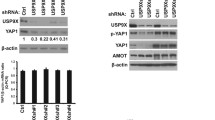
It has been shown that Yes-associated protein (YAP) acts as a transcriptional co-activator to regulate p73-dependent apoptosis in response to DNA damage in some cell types, and promyelocytic leukemia (PML) protein is involved in the regulation loop through stabilization of YAP through sumoylation. Although YAP has been shown to be significantly upregulated in gastric cancer, whether the YAP/PML/p73 regulation loop also functions in gastric cancer is unknown. Here, we show significantly higher levels of YAP and significantly lower levels of PML in the gastric cancer specimen. Overexpression of YAP in gastric cancer cells significantly increased cell growth, but did not affect apoptosis. However, overexpression of PML in gastric cancer cells significantly increased cell apoptosis, resulting in decreases in cell growth, which seemed to require the presence of YAP. The effect of PML on apoptosis appeared to be conducted through p73-mediated modulation of apoptosis-associated genes, Bcl-2, Bak, and caspase9. Thus, our study suggests the presence of a YAP/PML/p73 regulatory loop in gastric cancer, and highlights PML as a promising tumor suppressor in gastric cancer through YAP-coordinated cancer cell apoptosis.








Similar content being viewed by others
References
Zhao X, Li X, Yuan H. MicroRNAs in gastric cancer invasion and metastasis. Front Biosci. 2013;18:803–10.
Liu G, Jiang C, Li D, Wang R, Wang W. MiRNA-34a inhibits EGFR-signaling-dependent mmp7 activation in gastric cancer. Tumour Biol. 2014;35:9801–6.
Mao D, Zhang Y, Lu H, Zhang H. Molecular basis underlying inhibition of metastasis of gastric cancer by anti-vegfa treatment. Tumour Biol. 2014;35:8217–23.
Ye Y, Zhou X, Li X, Tang Y, Sun Y, Fang J. Inhibition of epidermal growth factor receptor signaling prohibits metastasis of gastric cancer via downregulation of MMp7 and MMp13. Tumour Biol. 2014;35:10891–6.
Wu W, Ding H, Cao J, Zhang W. Fbxl5 inhibits metastasis of gastric cancer through suppressing snail1. Cell Physiol Biochem. 2015;35:1764–72.
Xu XY, Zhang LJ, Yu YQ, Zhang XT, Huang WJ, Nie XC, et al. Down-regulated mac30 expression inhibits proliferation and mobility of human gastric cancer cells. Cell Physiol Biochem. 2014;33:1359–68.
Zhou X, Xia Y, Su J, Zhang G. Down-regulation of mir-141 induced by helicobacter pylori promotes the invasion of gastric cancer by targeting stat4. Cell Physiol Biochem. 2014;33:1003–12.
Cui Y, Chen J, He Z, Xiao Y. Suz12 depletion suppresses the proliferation of gastric cancer cells. Cell Physiol Biochem. 2013;31:778–84.
Mo JS, Park HW, Guan KL. The hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–56.
Zhou GX, Li XY, Zhang Q, Zhao K, Zhang CP, Xue CH, et al. Effects of the hippo signaling pathway in human gastric cancer. Asian Pac J Cancer Prev. 2013;14:5199–205.
Hao J, Zhang Y, Jing D, Li Y, Li J, Zhao Z. Role of hippo signaling in cancer stem cells. J Cell Physiol. 2014;229:266–70.
Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505.
Zhao Y, Khanal P, Savage P, She YM, Cyr TD, Yang X. Yap-induced resistance of cancer cells to antitubulin drugs is modulated by a hippo-independent pathway. Cancer Res. 2014;74:4493–503.
Hong W. Angiomotin’g yap into the nucleus for cell proliferation and cancer development. Sci Signal. 2013;6:pe27.
Reineke EL, Kao HY. Targeting promyelocytic leukemia protein: a means to regulating pml nuclear bodies. Int J Biol Sci. 2009;5:366–76.
Hodges M, Tissot C, Howe K, Grimwade D, Freemont PS. Structure, organization, and dynamics of promyelocytic leukemia protein nuclear bodies. Am J Hum Genet. 1998;63:297–304.
Fausti F, Di Agostino S, Cioce M, Bielli P, Sette C, Pandolfi PP, et al. ATM kinase enables the functional axis of YAP, PML and p53 to ameliorate loss of werner protein-mediated oncogenic senescence. Cell Death Differ. 2013;20:1498–509.
Okazaki T, Kageji T, Kuwayama K, Kitazato KT, Mure H, Hara K, et al. Up-regulation of endogenous PML induced by a combination of interferon-beta and temozolomide enhances p73/YAP-mediated apoptosis in glioblastoma. Cancer Lett. 2012;323:199–207.
Lapi E, Di Agostino S, Donzelli S, Gal H, Domany E, Rechavi G, et al. PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol Cell. 2008;32:803–14.
Downward J, Basu S. YAP and p73: a complex affair. Mol Cell. 2008;32:749–50.
Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, et al. The transcriptional coactivator yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol Cell. 2005;18:447–59.
Mei Q, Li F, Quan H, Liu Y, Xu H. Busulfan inhibits growth of human osteosarcoma through mir-200 family micrornas in vitro and in vivo. Cancer Sci. 2014;105:755–62.
Gurrieri C, Capodieci P, Bernardi R, Scaglioni PP, Nafa K, Rush LJ, et al. Loss of the tumor suppressor pml in human cancers of multiple histologic origins. J Natl Cancer Inst. 2004;96:269–79.
Gambacorta M, Flenghi L, Fagioli M, Pileri S, Leoncini L, Bigerna B, et al. Heterogeneous nuclear expression of the promyelocytic leukemia (PML) protein in normal and neoplastic human tissues. Am J Pathol. 1996;149:2023–35.
Lee HE, Jee CD, Kim MA, Lee HS, Lee YM, Lee BL, et al. Loss of promyelocytic leukemia protein in human gastric cancers. Cancer Lett. 2007;247:103–9.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Z., Chen, J., Shao, L. et al. Promyelocytic leukemia protein enhances apoptosis of gastric cancer cells through Yes-associated protein. Tumor Biol. 36, 8047–8054 (2015). https://doi.org/10.1007/s13277-015-3539-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3539-3