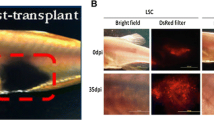
Abstract
Xenotransplantation studies are important tools for studying cancer biology, especially for assaying tumor cell malignancy and providing cancer information in vivo. Cancer stem-like cells (CSCs) have been identified in many cancer types to drive tumor growth and recurrence, from "keeping" to "keep" resistant to chemotherapy and radiation therapy. In this study, we developed the xenotransplantation of CSCs derived from the leukemia and solid tumor cell lines using the zebrafish models. In adult zebrafish, we investigated that the xenografted leukemia stem cells (LSCs) from K562 cells could proliferate in vivo and keep the cancer property by re-transplantation. As for the solid tumor, these CSCs from DU145 cells (human prostate cancer) and HepG2 cells (human liver cancer) could form the tumor mass and even metastasis after xenotransplantation. In addition, the zebrafish embryos with CSC xenotransplantation could evaluate docetaxel in vivo efficiently and be available to screen the novel inhibitors by high-throughput manner. In summary, these zebrafish xenotransplantation models devote a good platform for the CSC mechanism investigation and anti-CSC inhibitor screening.





Similar content being viewed by others
References
Jordan CT. The leukemic stem cell. Best Pract Res Clin Haematol. 2007;20:13–8.
Bachovchin DA, Brown SJ, Rosen H, Cravatt BF. Identification of selective inhibitors of uncharacterized enzymes by high-throughput screening with fluorescent activity-based probes. Nat Biotechnol. 2009;27:387–94.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67.
Hellsten R, Johansson M, Dahlman A, Sterner O, Bjartell A. Galiellalactone inhibits stem cell-like ALDH-positive prostate cancer cells. PLoS ONE. 2011;6:e22118.
Fleischman AG. ALDH marks leukemia stem cell. Blood. 2012;119:3376–7.
Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res: MCR. 2008;6:1146–53.
Taylor AM, Zon LI. Zebrafish tumor assays: the state of transplantation. Zebrafish. 2009;6:339–46.
Kruczynski A, Hill BT: Classic in vivo cancer models: three examples of mouse models used in experimental therapeutics. Curr Protoc Pharmacol. 2002. Chapter 5: Unit5. 24.
Goldsmith P. Zebrafish as a pharmacological tool: the how, why and when. Curr Opin Pharmacol. 2004;4:504–12.
Amatruda JF, Shepard JL, Stern HM, Zon LI. Zebrafish as a cancer model system. Cancer Cell. 2002;1:229–31.
Yang XJ, Cui W, Gu A, Xu C, Yu SC, Li TT, et al. A novel zebrafish xenotransplantation model for study of glioma stem cell invasion. PLoS ONE. 2013;8:e61801.
Zhang B, Shimada Y, Kuroyanagi J, Umemoto N, Nishimura Y, Tanaka T. Quantitative phenotyping-based in vivo chemical screening in a zebrafish model of leukemia stem cell xenotransplantation. PLoS ONE. 2014;9:e85439.
White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–9.
Peal DS, Peterson RT, Milan D. Small molecule screening in zebrafish. J Cardiovasc Transl Res. 2010;3:454–60.
Nicoli S, Ribatti D, Cotelli F, Presta M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007;67:2927–31.
Marques IJ, Weiss FU, Vlecken DH, Nitsche C, Bakkers J, Lagendijk AK, et al. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer. 2009;9:128.
Dovey MC, Zon LI. Defining cancer stem cells by xenotransplantation in zebrafish. Methods Mol Biol. 2009;568:1–5.
Ignatius MS, Langenau DM. Zebrafish as a model for cancer self-renewal. Zebrafish. 2009;6:377–87.
Yang IH, Lee D, Lee SH, Kang JY. Characterization of proteolytically digested zebrafish chorion as extracellular matrix. Conf Proc: IEEE Eng Med Biol Soc. 2008;2008:1837–40.
Zang L, Shimada Y, Nishimura Y, Tanaka T, Nishimura N. A novel, reliable method for repeated blood collection from aquarium fish. Zebrafish. 2013;10:425–32.
Eguiara A, Holgado O, Beloqui I, Abalde L, Sanchez Y, Callol C, et al. Xenografts in zebrafish embryos as a rapid functional assay for breast cancer stem-like cell identification. Cell Cycle. 2011;10:3751–7.
Rohena CC, Mooberry SL. Recent progress with microtubule stabilizers: new compounds, binding modes and cellular activities. Nat Prod Rep. 2014;31:335–55.
Lee C, Wu SS, Chen LB. Photosensitization by 3,3ʹ-dihexyloxacarbocyanine iodide: specific disruption of microtubules and inactivation of organelle motility. Cancer Res. 1995;55:2063–9.
Rowinsky EK, Onetto N, Canetta RM, Arbuck SG. Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol. 1992;19:646–62.
Arbuck SG, Christian MC, Fisherman JS, Cazenave LA, Sarosy G, Suffness M, et al. Clinical development of taxol. J Natl Cancer Inst Monogr. 1993;15:11–24.
Foa R, Norton L, Seidman AD. Taxol (paclitaxel): a novel anti-microtubule agent with remarkable anti-neoplastic activity. Int J Clin Lab Res. 1994;24:6–14.
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3.
Rudin CM, Salgia R, Wang X, Hodgson LD, Masters GA, Green M, et al. Randomized phase II study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26:870–6.
Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–64.
Haldi M, Ton C, Seng WL, McGrath P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis. 2006;9:139–51.
Traver D, Herbomel P, Patton EE, Murphey RD, Yoder JA, Litman GW, et al. The zebrafish as a model organism to study development of the immune system. Adv Immunol. 2003;81:253–330.
Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–46.
Pichler FB, Laurenson S, Williams LC, Dodd A, Copp BR, Love DR. Chemical discovery and global gene expression analysis in zebrafish. Nat Biotechnol. 2003;21:879–83.
Acknowledgments
We thank M. Ariyoshi and S. Ichikawa for their assistance in the experiments and breeding the fish and R. Ikeyama and Y. Tamura for the secretarial assistance.
Conflicts of interest
None
Funding sources
This work was supported in part by the Japan Science and Technology Agency, KAKENHI of Grants-in-Aid for Scientific Research, and the New Energy and Industrial Technology Development Organization.
Author contribution
T.T. and Y.S. conceived and designed the study. B.Z., J.K., Y.S., N.U., and Y.N. performed the assays and analyzed the data. T.N, T.S., and T.M. performed chemical synthesis assays. B.Z. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Different cancer processes of LSC and NC xenografts. (a) K562 cells expressed Kusabira-Orange (KOr) fluorescent protein after transfection. (b) KOr positive cells (indicated by yellow arrows) in the blood from LSC and NC xenotransplanted zebrafish at 35 dpi. (c) The agarose gel electrophoresis for KOr gene in the blood of xenografted fish (35 dpi). C, Control fish without cancer injection, L, LSCs injected zebrafish, N, NCs injected zebrafish, M, Marker. (d) The agarose gel electrophoresis for kor gene after 10 days culture of the KOr positive cells after isolation from fish blood. (PDF 247 kb)
Fig. S2
CSC proliferations were inhibited by chemicals in vitro. (a) Docetaxel inhibited ALDH + cells of DU145-KOr after 24 h exposure at different doses. n = 4, **P < 0.01. (b) One fluorescent chemical B647 inhibited ALDH + cells of K562-BFP after 24 h exposure at different doses. n = 4, **P < 0.01. (PDF 166 kb)
Rights and permissions
About this article
Cite this article
Zhang, B., Shimada, Y., Kuroyanagi, J. et al. Zebrafish xenotransplantation model for cancer stem-like cell study and high-throughput screening of inhibitors. Tumor Biol. 35, 11861–11869 (2014). https://doi.org/10.1007/s13277-014-2417-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2417-8