Abstract
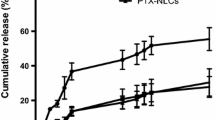
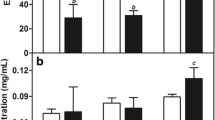
Breast cancer is the most prevalent cancer among women. Recently, delivering by nanocarriers has resulted in a remarkable evolution in treatment of numerous cancers. Lipid nanocarriers are important ones while liposomes and archaeosomes are common lipid nanocarriers. In this work, paclitaxel was used and characterized in nanoliposomal and nanoarchaeosomal form to improve efficiency. To increase stability, efficiency and solubility, polyethylene glycol 2000 (PEG 2000) was added to some samples. MTT assay confirmed effectiveness of nanocarriers on MCF-7 cell line and size measuring validated nano-scale of particles. Nanoarchaeosomal carriers demonstrated highest encapsulation efficiency and lowest release rate. On the other hand, pegylated nanoliposomal carrier showed higher loading efficiency and less release compared with nanoliposomal carrier which verifies effect of PEG on improvement of stability and efficiency. Additionally, release pattern was modeled using artificial neural network (ANN) and genetic algorithm (GA). Using ANN modeling for release prediction, resulted in R values of 0.976, 0.989 and 0.999 for nanoliposomal, pegylated nanoliposomal and nanoarchaeosomal paclitaxel and GA modeling led to values of 0.954, 0.951 and 0.976, respectively. ANN modeling was more successful in predicting release compared with the GA strategy.












Similar content being viewed by others
References
Guo J, Bourre L, Soden DM, O'Sullivan GC, O'Driscoll C. Can non-viral technologies knockdown the barriers to siRNA delivery and achieve the next generation of cancer therapeutics? Biotechnol Adv. 2011;29:402–17.
Blagosklonny MV. A node between proliferation, apoptosis, and growth arrest. Bioessays. 1999;21:704–9.
Rostas JW, Dyess DL. Current operative management of breast cancer: an age of smaller resections and bigger cures. Int J Breast Cancer. 2012;2012:516417.
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7.
Brasnjevic I, Steinbusch HW, Schmitz C, Martinez-Martinez P. European NanoBioPharmaceutics Research Initiative. Delivery of peptide and protein drugs over the blood–brain barrier. Prog Neurobiol. 2009;87:212–51.
Dorr RT. Pharmacology and toxicology of Cremophor EL diluent. Ann Pharmacother. 1994;28:11–4.
Sprott G. Structures of archaebacterial membrane lipids. J Bioenerg Biomembr. 1992;24:555–66.
Dayhoff JE. Neural network principles. 1st ed. Prentice Hall: USA; 1990.
Neocleous C, Schizas C. Artificial neural network learning: a comparative review. 1st ed. Berlin: Springer; 2002.
Richard BD, Liu YA. Neural network in bioprocessing and chemical engineering. 1st ed. USA: Academic Press; 1996.
Terfloth L, Gasteiger J. Neural networks and genetic algorithms in drug design. Genomics Suppl. 2012;6:102–8.
Mukhopadhyay DM, Balitanas MO, Farkhod AA, Jeon SH, Bhattacharyya D. Genetic algorithm: a tutorial review. Int J Grid Distrib Comput. 2009;2:25–32.
Graupe D. Principles of ANN. 2nd ed. Singapore: World Scientific Publishing; 2007.
Krishnamachari Y, Geary SM, Lemke CD, Salem AK. Nanoparticle delivery systems in cancer vaccines. Pharm Res. 2011;28:215–36.
Haykin S. Neural networks: a comprehensive foundation. 2nd ed. Prentice Hall: USA; 1998.
Laugier S, Richon D. Use of artificial neural networks for calculating derived thermodynamic quantities from volumetric property data. Fluid Phase Equilib. 2003;2:247–55.
Rizkalla N, Hildgen P. Artificial neural networks: Comparison of two programs for modeling a process of nanoparticle preparation. Drug Dev Ind Pharm. 2005;31:1019–33.
Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK, et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 2007;338:317–26.
Zhang JA, Anyarambhatla G, Ma L, Ugwu S, Xuan T, Sardone T, et al. Development and characterization of a novel Cremophor EL free liposome-based paclitaxel (LEP-ETU) formulation. Eur J Pharm Biopharm. 2005;59:177–87.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Movahedi, F., Ebrahimi Shahmabadi, H., Alavi, S.E. et al. Release modeling and comparison of nanoarchaeosomal, nanoliposomal and pegylated nanoliposomal carriers for paclitaxel. Tumor Biol. 35, 8665–8672 (2014). https://doi.org/10.1007/s13277-014-2125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2125-4