Abstract
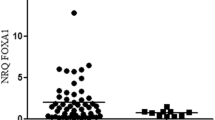
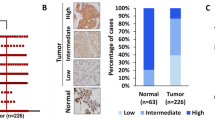
This study aimed to analyze the expression, clinical significance of epithelial membrane protein 1 (EMP1) in breast carcinoma and the biological effect in its cell line by EMP1 overexpression. Immunohistochemistry and western blot were used to analyze EMP1 protein expression in 67 cases of breast cancer and 35 cases of normal tissues to study the relationship between EMP1 expression and clinical factors. Quantitative real-time RT-PCR and western blot were used to detect the mRNA level and protein of EMP1. MTT assay, migration and invasion assays were also conducted as to the influence of the upregulated expression of EMP1 that might be found on MCF-7 cell biological effect. The relative amount of EMP1 protein in breast cancer tissue was found to be significantly lower than in normal tissues (P < 0.05). The level of EMP1 protein expression was correlated with T stages, lymph node metastasis, clinic stage, and histological grade (P < 0.05). Meanwhile, loss of EMP1 expression correlated significantly with poor overall survival time by Kaplan–Meier analysis (P < 0.05). The result shown that MCF-7 cell transfected EMP1 had a lower survival fraction, higher cell apoptosis, significant decrease in migration and invasion, higher caspase-9, and lower VEGFC protein expression compared with MCF-7 cell untransfected EMP1 (P < 0.05). EMP1 expression decreased in breast cancer and correlated significantly with lymph node metastasis, clinic stage, histological grade, and poor overall survival, T stages, suggesting that EMP1 may play important roles as a negative regulator to breast cancer MCF-7 cell by regulating the expression of caspase 9 and VEGFC protein.








Similar content being viewed by others
References
Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36:237–48.
Stuckey A. Breast cancer: epidemiology and risk factors. Clin Obstet Gynecol. 2011;54:96–102.
Lai S, Wang G, Cao X, Li Z, Hu J, Wang J. EMP-1 promotes tumorigenesis of NSCLC through PI3K/AKT pathway. J Huazhong Univ Sci Technolog Med Sci. 2012;32:834–8.
Taylor V, Welcher AA, Program AE, Suter U. Epithelial membrane protein-1, peripheral myelin protein 22, and lens membrane protein 20 define a novel gene family. J Biol Chem. 1995;270:28824–33.
Lobsiger CS, Magyar JP, Taylor V, Wulf P, Welcher AA, Program AE, et al. Identification and characterization of a cDNA and the structural gene encoding the mouse epithelial membrane protein-1. Genomics. 1996;36:379–87.
Wulf P, Suter U. Embryonic expression of epithelial membrane protein 1 in early neurons. Brain Res Dev Brain Res. 1999;116:169–80.
Zoidl G, Blass-Kampmann S, D’Urso D, Schmalenbach C, Müller HW. Retroviral-mediated gene transfer of the peripheral myelin protein PMP22 in Schwann cells: modulation of cell growth. EMBO J. 1995;14:1122–8.
Jetten AM, Suter U. The peripheral myelin protein 22 and epithelial membrane protein family. Prog Nucleic Acid Res Mol Biol. 2000;64:97–129.
Lee HS, Sherley JL, Chen JJ, Chiu CC, Chiou LL, Liang JD, et al. EMP1 is a junctional protein in a liver stem cell line and in the liver. Biochem Biophys Res Commun. 2005;334:996–1003.
Gnirke AU, Weidle UH. Investigation of prevalence and regulation of expression of progression associated protein (PAP). Anticancer Res. 1998;18:4363–9.
Wang HT, Kong JP, Ding F, Wang XQ, Wang MR, Liu LX, et al. Analysis of gene expression profile induced by EMP-1 in esophageal cancer cells using cDNA microarray. World J Gastroenterol. 2003;9:392–8.
Zhang J, Cao W, Xu Q, Chen WT. The expression of EMP1 is downregulated in oral squamous cell carcinoma and possibly associated with tumour metastasis. J Clin Pathol. 2011;64:25–9.
Yoshimoto M, Tada K, Hori H, Morota A, Tanabe M, Nishimura S, et al. Improvement in the prognosis of Japanese breast cancer patients from 1946 to 2001—an institutional review. Jpn J Clin Oncol. 2004;34:457–62.
Schnitt SJ. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2009;201023:S60–4.
Lin DC, Du XL, Wang MR. Protein alterations in ESCC and clinical implications: a review. Dis Esophagus. 2009;22:9–20.
Escobar PF, Patrick RJ, Rybicki LA, Weng DE, Crowe JP. The 2003 revised TNM staging system for breast cancer: results of stage re-classification on survival and future comparisons among stage groups. Ann Surg Oncol. 2007;14:143–7.
Sen S, Kawahara B, Chaudhuri G. Mitochondrial-associated nitric oxide synthase activity inhibits cytochrome c oxidase: implications for breast. Cancer Free Radic Biol Med. 2013;57:210–20.
Zhang JM, Wang HC, Wang HX, Ruan LH, Zhang YM, Li JT, et al. Oxidative stress and activities of caspase-8, -9, and -3 are involved in cryopreservation-induced apoptosis in granulosa cells. Eur J Obstet Gynecol Reprod Biol. 2013;166:52–5.
Peng J, Shao N, Peng H, Chen LQ. Prognostic significance of vascular endothelial growth factor expression in esophageal carcinoma: a meta-analysis. J Buon. 2013;18:398–406.
Xie LX, Zhai TT, Yang LP, Yang E, Zhang XH, Chen JY, et al. Lymphangiogenesis and prognostic significance of vascular endothelial growth factor C in gastro-oesophageal junction adenocarcinoma. Int J Exp Pathol. 2013;94:39–46.
Acknowledgments
This research was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, G.G., Wang, Y.D., Lu, Y.F. et al. EMP1, a member of a new family of antiproliferative genes in breast carcinoma. Tumor Biol. 35, 3347–3354 (2014). https://doi.org/10.1007/s13277-013-1441-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1441-4