Abstract
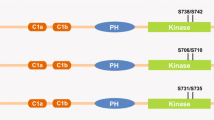
Cyclic GMP-dependent protein kinases (PKG) constitute a small family of enzymes that are encoded by two genes. Two major forms of PKG have been identified in mammalian cells, PKG I and PKG II. In addition, there are two splice variants of PKG I, which are designated as Iα and Iβ. There are increasing evidences that PKG can play an important role in the inhibition of cell proliferation and induction of apoptosis. In our previous studies, the inhibitory effects of cGMP/PKG on the cell growth were indicated using breast cancer cell lines. Accordingly, the present study was designed to compare the expression levels of three PKG isoforms in normal, benign, and malignant breast tissues. The expression level of PKG isoforms was assayed using quantitative real-time RT-PCR. The correlation between relative expression of PKG isoforms and clinicopathological characteristics were also analyzed. Downregulation of PKG isoforms was observed in the malignant and benign tumors when compared to those of respective normal tissues. No significant correlation was found between PKGIα, PKGIβ, and PKGII expression and clinicopathological features. The present study is the first to evaluate the expression level of PKG isoforms PKGIα, PKGIβ, and PKGII in the malignant and benign breast tumors. Reduction in the PKG expression is an important evidence to support the antitumor activity of this enzyme in vivo.


Similar content being viewed by others
Change history
22 October 2021
An addendum to this article is available online at https://doi.org/10.3233/TUB-219006
References
Fiscus RR, Murad F. cGMP-dependent protein kinase activation in intact tissues. Methods Enzymol. 1988;159:150–9.
Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMPdependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86:1–23.
Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP dependent protein kinase. Front Biosci. 2006;11:356–67.
Orstavik S, Natarajan V, Tasken K, Jahnsen T, Sandberg M. Characterization of the human gene encoding the type I alpha and type I beta cGMP-dependent protein kinase (PRKG1). Genomics. 1997;42:311–8.
Orstavik S, Solberg R, Tasken K, Nordahl M, Altherr MR, Hansson V, Jahnsen T, Sandberg M. Molecular cloning, cDNA structure and chromosomal localization of the human type cGMP-dependent protein kinase. Biochem Biophys res Commun. 1996;220:759–65.
Gamm DM, Francis SH, Angelotti TP, Uhler MD. The type isoform of cGMP-dependent protein kinase is dimeric and possesses regulatory and catalytic properties distinct from the type isoforms. J Biol chem. 1995;270:27380–8.
Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci. 1997;22:307–12.
Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–30.
Lohmann SM, Fischmeister R, Walter U. Signal transduction by cGMP in heart. Basic Res Cardiol. 1991;86:503–14.
Uhler MD. Cloning and expression of a novel cyclic GMP-dependent protein kinase from mouse brain. J Biol Chem. 1993;268:13586–91.
Shimojo T, Hiroe M, Ishiyama S, Ito H, Nishikawa T, Marumo F. Nitric oxide induces apoptotic death of cardiomyocytes via a cyclic-GMP-dependent pathway. Exp Cell Res. 1999;247:38–47.
Loweth AC, Williams GT, Scarpello JNB, Morgan NG. Evidence for the involvement of cGMP and protein kinase G in nitric oxide-induced apoptosis in the pancreatic B-cell line, HIT-T15. FEBS Lett. 1997;400:285–8.
Kaminski A, Gao H, Morgan NG. Involvement of the cGMP signalling pathway in the regulation of viability in insulin-secreting BRIN-BD11 cells. FEBS Lett. 2004;559:118–24.
Cook AL, Haynes JM. Protein kinase G II-mediated proliferative effects in human cultured prostatic stromal cells. Cell Signal. 2004;16:253–61.
Chiche JD, Schlutsmeyer SM, Bloch DB, De la Monte SM, Roberts JD. Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J Biol Chem. 1998;273:34263–71.
Deguchi A, Thompson WJ, Weinstein IB. Activation of protein kinase G is sufficient to induce apoptosis and inhibit cell migration in colon cancer cells. Cancer Res. 2004;64:3966–73.
Browning DD. cGMP-dependent protein kinases as potential targets for colon cancer prevention and treatment. Future Med Chem. 2010;2:65–80.
Browning DD. Protein kinase G as a therapeutic target for the treatment of metastatic colorectal cancer. Expert Opin Biol Ther. 2008;8:1–10.
Fallahian F, Karami-Tehrani F, Salami S, Aghaei M. Cyclic GMP induced apoptosis via protein kinase G in oestrogen receptor-positive and -negative breast cancer cell lines. FEBS J. 2011;278:3360–9.
Fallahian F, Karami-Tehrani F, Salami S. Induction of apoptosis by type Iβ protein kinase G in the human breast cancer cell lines MCF-7 and MDA-MB-468. Cell Biochem Func. 2012;30:183–90.
Soh JW, Mao Y, Kim MG, Pamukcu R, Li H, Piazza GA, Thompson WJ, Weinstein IB. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin Cancer Res. 2000;6:4136–41.
Soh JW, Mao Y, Liu L, Thompson WJ, Pamukcu R, Weinstein IB. Protein kinase G activates the JNK1 pathway via phosphorylation of MEKK1. J Biol Chem. 2001;276:16406–10.
Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. Exisulind induction of apoptosis involves guanosine 3 ′,5 ′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338–42.
Lin G, Chow S, Lin J, Wang G, Lue TF, Lin CS. Effect of cell passage and density on protein kinase G expression and activation in vascular smooth muscle cells. J Cell Biochem. 2004;92:104–12.
Cornwell TL, Lincoln TM. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem. 1989;264:1146–55.
Vaandrager AB, Tilly BC, Smolenski A, Schneider-Rasp S, Bot AG, Edixhoven M, Scholte BJ, Jarchau T, Walter U, Lohmann SM, Poller WC, de Jonge HR. cGMP stimulation of cystic fibrosis transmembrane conductance regulator Cl– channels co-expressed with cGMP-dependent protein kinase type II but not type Iβ. J Biol Chem. 1997;272:4195–200.
Hou Y, Gupta N, Schoenlein P, Wong E, Martindale R, Ganapathy V, Browning DD. An anti-tumor role for cGMP-dependent protein kinase. Cancer Lett. 2006;240:60–8.
Leung EL, Wong JC, Johlfs MG, Tsang BK, Fiscus RR. Protein kinase G type Ialpha activity in human ovarian cancer cells significantly contributes to enhanced Src activation and DNA synthesis/cell proliferation. Mol Cancer Res. 2010;8:578–91.
Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, Piazza GA. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–40.
Deguchi A, Das KK, Xing SW, Oehlen B, Weinstein IB (2005) Down-regulation of the cGMP/PKG pathway in primary human colon cancers and cancer cell lines. Experimental and Molecular Therapeutics 20 (Abstract)
Kwon IK, Schoenlein PV, Delk J, Liu K, Thangaraju M, Dulin NO, Ganapathy V, Berger FG, Browning DD. Expression of cyclic guanosine monophosphate-dependent protein kinase in metastatic colon carcinoma cells blocks tumor angiogenesis. Cancer. 2008;112:1462–70.
Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/β-catenin-mediated transcription in human breast tumor cells. Cancer Prev Res (Phila). 2011;4:1275–84.
Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karami-Tehrani, F., Fallahian, F. & Atri, M. Expression of cGMP-dependent protein kinase, PKGIα, PKGIβ, and PKGII in malignant and benign breast tumors. Tumor Biol. 33, 1927–1932 (2012). https://doi.org/10.1007/s13277-012-0453-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0453-9