Abstract
Background
Cigarette smoke-induced chronic airway inflammation increases the risk of lung cancer and plays a multifaceted role in lung cancer initiation and progression. The RAGE-ligand axis (HMGB1, S100A8/A9) contributes to cigarette smoke-induced persistent and progressive inflammation. The aim of the present study was to determine the inflammatory effect on the S100A8/A9-RAGE pathway in smoke-induced lung cancer carcinogenesis.
Methods
Human alveolar adenocarcinoma cells (A549) and normal bronchial epithelial cells (BEAS-2B) were co-cultured for 24 h with PBMCs and then were incubated in the presence or absence of 1.5% cigarette smoke extract (CSE) for 24 h. The cells were then transfected with siRNA targeting S100A8/9 or RAGE. Cell viability, colony-forming ability, migration, invasion, metastasis, and morphological changes were assessed. Female A/J mice were given benzo(a)pyrene (B(a)P; 100 mg/kg) in 0.1 mL of corn oil via oral gavage once a week for 3 weeks and administrated CSE (1.25 ul/g) intratracheally twice a week for 4 weeks. Tumor load was determined by averaging the total tumor volume in each group. Bronchoalveolar lavage (BAL) cells were differentiated and counted to analyze inflammatory cells present.
Results
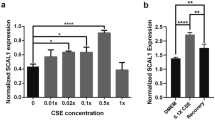
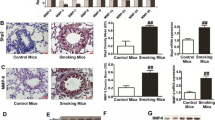
Cell growth and colony formation were promoted by exposure to CSE and significantly inhibited by S100A8/9 and RAGE-siRNA transfection, especially in A549 cells compared to BEAS-2B cells. Cell migration, invasion, MMP-2/9 activity, and the mRNA and protein expression of TLR4, NF-κB, RAGE, S100A8/9, and HMBG1 were increased in CSE-exposed A549 cells. S100A8/9 and RAGE-siRNA transfection significantly attenuated those expressions. B(a)P induced an average tumor volume of 7.28 mm3 per mouse, and CSE significantly increased the tumor volume (8.10 mm3) compared to the B(a)P group. The total cell number and lymphocytes in BAL fluid tended to increase after CSE administration in the lung cancer mouse group. Moreover, CSE significantly induced the levels of S100A8/9, RAGE, p38, and ERK, and decreased JNK in serum and tumors compared to adjacent lung tissues (p < 0.05).
Conclusion
Our study suggests that the S100A8/A9-RAGE signal pathway is the mainstream of the inflammation-immune response induced in cancer progression in CSE-related smoke-induced lung carcinogenesis in vitro and in vivo. Further studies are needed to identify the therapeutic targets of cigarette smoke-induced inflammation and immunosuppression in the tumor microenvironment.





Similar content being viewed by others
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PBMC:
-
Peripheral blood mononuclear cell
- TLR4:
-
Toll-like receptor 4
- NF-κB:
-
Nuclear factor kappa-light-chain enhancer of activated B cells
- RAGE:
-
Receptor for advanced glycation end products
- HMBG1:
-
High mobility group box 1
- ERK:
-
Extracellular signal-regulated kinase
- JNK:
-
C-Jun N-terminal kinase
- MMP:
-
Matrix metalloproteinase
References
Adcock IM, Caramori G, Barnes PJ (2011) Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration 81:265–284
Cho WC et al (2011) The role of inflammation in the pathogenesis of lung cancer. Expert Opin Ther Targets 15:1127–1137
De Flora S et al (2016) Pharmacological modulation of lung carcinogenesis in smokers: preclinical and clinical evidence. Trends Pharmacol Sci 37:120–142
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ (2010) HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28:367–388
Li M et al (2015) RAGE-ligands axis: a new “driving force” for cigarette smoke-induced airway inflammation in COPD? Respirology 20:998–999
Li JJN et al (2020) Tobacco exposure and immunotherapy response in PD-L1 positive lung cancer patients. Lung Cancer 150:159–163
Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 59:1593–1600
Cheng P et al (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205:2235–2249
Chen M et al (2017) Knockout of RAGE ameliorates mainstream cigarette smoke-induced airway inflammation in mice. Int Immunopharmacol 50:230–235
Oczypok EA, Perkins TN, Oury TD (2017) All the “RAGE” in lung disease: the receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev 23:40–49
Ortiz ML, Lu L, Ramachandran I, Gabrilovich DI (2014) Myeloid-derived suppressor cells in the development of lung cancer. Cancer Immunol Res 2:50–58
Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM (2009) Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst 101:554–559
Ramasamy R, Yan SF, Schmidt AM (2007) Arguing for the motion: yes, RAGE is a receptor for advanced glycation endproducts. Mol Nutr Food Res 51:1111–1115
Chen B et al (2015) S100A9 induced inflammatory responses are mediated by distinct damage associated molecular patterns (DAMP) receptors in vitro and in vivo. PLoS ONE 10:e0115828
Lee H, Lee J, Hong SH, Rahman I, Yang SR (2018) Inhibition of RAGE attenuates cigarette smoke-induced lung epithelial cell damage via RAGE-mediated Nrf2/DAMP signaling. Front Pharmacol 9:684
Cheng Y et al (2017) HMGB1 translocation and release mediate cigarette smoke-induced pulmonary inflammation in mice through a TLR4/MyD88-dependent signaling pathway. Mol Biol Cell 28:201–209
Lee H et al (2017) Blockade of RAGE ameliorates elastase-induced emphysema development and progression via RAGE-DAMP signaling. FASEB J 31:2076–2089
Yang IA et al (2011) Common pathogenic mechanisms and pathways in the development of COPD and lung cancer. Expert Opin Ther Targets 15:439–456
Gomes M, Teixeira AL, Coelho A, Araujo A, Medeiros R (2014) The role of inflammation in lung cancer. Adv Exp Med Biol 816:1–23
Chapman S et al (2018) Cigarette smoke extract induces oral squamous cell carcinoma cell invasion in a receptor for advanced glycation end-products-dependent manner. Eur J Oral Sci 126:33–40
Sanders NT et al (2017) Cigarette smoke extract (CSE) induces RAGE-mediated inflammation in the Ca9-22 gingival carcinoma epithelial cell line. Arch Oral Biol 80:95–100
Srikrishna G (2012) S100A8 and S100A9: new insights into their roles in malignancy. J Innate Immun 4:31–40
Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G (2011) S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res 9:133–148
Tomonobu N, Kinoshita R, Sakaguchi M (2020) S100 soil sensor receptors and molecular targeting therapy against them in cancer metastasis. Transl Oncol 13:100753
Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ (2016) Tumor-infiltrating monocytes/macrophages promote tumor invasion and migration by upregulating S100A8 and S100A9 expression in cancer cells. Oncogene 35:5735–5745
Nedjadi T et al (2018) S100A8 and S100A9 proteins form part of a paracrine feedback loop between pancreatic cancer cells and monocytes. BMC Cancer 18:1255
Wang T et al (2018) A review of S100 protein family in lung cancer. Clin Chim Acta 476:54–59
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2020R1F1A1066281).
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2020R1F1A1066281).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Sung Bae Cho declares that he/she has no conflict of interest. In Kyoung declares that he/she has no conflict of interest. Hye Seon Kang declares that he/she has no conflict of interest. Sang Haak Lee that he/she has no conflict of interest. Chang Dong Yeo declares that he/she has no conflict of interest.
Ethical approval
This study was approved by the Ethical Committee on Animal Experiments of the Catholic University of Korea (EPSMH-2020-30-02-H).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, S.B., Kim, I.K., Kang, H.S. et al. S100A8/A9-RAGE pathway and chronic airway inflammation in smoke-induced lung carcinogenesis. Mol. Cell. Toxicol. 20, 177–186 (2024). https://doi.org/10.1007/s13273-023-00339-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00339-0