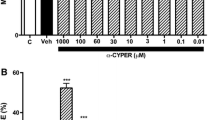
Abstract
Copper oxide nanoparticles (CuO NPs) are one of the most toxic metal oxide nanoparticles, but they have a wide range of applications in the manufacture of industrial and consumer products. While their beneficial aspects are widely acknowledged, the toxic effects of CuO NPs and Cu ions on the neuronal system may limit their use. Here, we propose a molecular mechanism underlying the response of human neuroblastoma SH-SY5Y cells to CuO NP and Cu ion exposure based on cDNA microarray data. SH-SY5Y cells exposed to CuO NPs and CuCl2 for 24 hours showed concentration-dependent cell death with similar IC50 values. The expression level of up-regulated genes in CuCl2-exposed cells was significantly higher than in CuO NP-exposed cells. CuO NP-exposed cells arrested the cell cycle in the G1/S/G2/M phases as a result of an up-regulation of INK4d, CYCE, RB, CDC6, CDC45, GADD45, and BUB1, while CuCl2-exposed cells mostly arrested the cell cycle in the G2/M phases as a result of CYCA, CYCB, CDK1, CDC20, PIK1, and BUB1 up-regulation. Our results suggest that the IC50 value had no significant relation with gene expression changes because the gene expression study may be in part due to cytotoxic mechanisms.
Similar content being viewed by others
References
Pal, J. & Pal, T. Faceted metal and metal oxide nanoparticles: design, fabrication and catalysis. Nanoscale 7, 14159–14190 (2015).
Sun, Y.F. et al. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 12, 2610–2631 (2012).
Badawy, W.A. et al. A review on solar cells from Sisingle crystals to porous materials and quantum dots. J. Adv. Res. 6, 123–132 (2015).
Kidowaki, H. et al. Fabrication and characterization of CuO-based solar cells. J. Mat. Sci. Res. 1, 138–143 (2012).
Gupta, K. et al. Synthesis of La 0.67 Sr 0.33 MnO3 and polyaniline nanocomposite with its electrical and magneto-transport properties. J. Appl. Phys. 107, 073704 (2010).
Sundar, S. & Prajapati, V.K. et al. Drug targeting to infectious diseases by nanoparticles surface functionalized with special biomolecules. Curr. Med. Chem. 19, 3196–3202 (2012).
Chang, Y.N. et al. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5, 2850–2871 (2012).
Bondarenko, O. et al. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch. Toxicol. 87, 1181–1200 (2013).
Zhou, K. et al. Synthesis, characterization and catalytic properties of CuO nanocrystals with various shapes. Nanotechnology 17, 3939–3943 (2006).
Chang, H. et al. Rheology of CuO nanoparticle suspension prepared by ASNSS. Rev. Adv. Mater. Sci. 10, 128–132 (2005).
Adamcakova-Dodd, A. et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part. Fibre Toxicol. 11, 15 (2014).
Guo, X. & Chen, T. Progress in genotoxicity evaluation of engineered nanomaterials. INTECH, DOI: 10.5772/61013, 141–160 (2015).
Bondarenko, O. et al. Sub-toxic effects of CuO nanoparticles on bacteria: Kinetics, role of Cu ions and possible mechanisms of action. Environ. Pollut. 169, 81–89 (2012).
Aruoja, V. et al. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 407, 1461–1468 (2009).
Shafagh, M. et al. CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and P53. Iran. J. Basic Med. Sci. 18, 993–1000 (2015).
Nations, S. et al. Acute effects of Fe2O3, TiO2, ZnO and CuO nanomaterials on Xenopus laevis. Chemosphere 83, 1053–1061 (2011).
Sandhya Rani, V. et al. Pulmonary toxicity of Copper oxide (CuO) nanoparticles in rats. J. Med. Sci. 13, 571–577 (2013).
Hanagata, N. et al. Molecular responses of human lung epithelial cells to the toxicity of copper oxide nanoparticles inferred from whole genome expression analysis. ACS Nano 5, 9326–9338 (2011).
Jomova, K. & Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 283, 65–87 (2011).
Wang, Z. et al. Biological and environmental transformations of Copper-based nanomaterials. ACS Nano 7, 8715–8727 (2013).
Xu, L. et al. Genotoxicity and molecular response of silver nanoparticle (NP)-based hydrogel. J. Nanobiotechnology 10, 16 (2012).
Kumaran, R.S. et al. Quantitation of oxidative stress gene expression in MCF-7 human cell lines treated with water-dispersible CuO nanoparticles. Appl. Biochem. Biotechnol. 173, 731–740 (2014).
Siddiqui, M.A. et al. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLOS ONE 8, e69534 (2013).
Li, F. et al. High content image analysis for human H4 neuroglioma cells exposed to CuO nanoparticles. BMC Biotechnol. 7, 66 (2007).
Yang, Y. et al. Global gene expression analysis of the effects of gold nanoparticles on human dermal fibroblasts. J. Biomed. Nanotechnol. 6, 234–246 (2010).
Masseroli, M. & Pinciroli, F. Using gene ontology and genomic controlled vocabularies to analyze highthroughput gene lists: Three tool comparison. Comput. Biol. Med. 36, 731–747 (2006).
Kim, J.A. et al. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat. Nanotechnol. 7, 62–68 (2012).
Cunningham, J.J. et al. The cyclin-dependent kinase inhibitors p19Ink4d and p27Kip1 are coexpressed in select retinal cells and act cooperatively to control cell cycle exit. Mol. Cell Neurosci. 19, 359–374 (2002).
Tourigny, M.R. et al. CDK inhibitor p18INK4c is required for the generation of functional plasma cells. Immunity 17, 179–189 (2002).
Garcia-Bustos, J.F. et al. PIK1, an essential phosphatidylinositol 4-kinase associated with the yeast nucleus. EMBO J. 13, 2352–2361 (1994).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jang, SW., Oh, MS., Yang, S.I. et al. Gene expression profiles of human neuroblastoma cells exposed to CuO nanoparticles and Cu ions. BioChip J 10, 140–149 (2016). https://doi.org/10.1007/s13206-016-0209-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-016-0209-5