Abstract
Darunavir, an anti-HIV drug having poor solubility in aqueous and lipid medium, illustrates degradation above its melting point, i.e. 74 °C, thus, posing a challenge to dosage formulation. Despite, the drug suffers from poor oral bioavailability (37%) owing to less permeability and being poly-glycoprotein and cyp3A metabolism substrate. The study aimed formulating a SLN system to overcome the formulation and bioavailability associated problems of the drug. Based on the drug solubility and stable dispersion findings, lipid and surfactant were chosen and nanoparticles were prepared using hot-homogenization technique. Optimization of variables such as lipid concentration, oil-surfactant and homogenization cycle was carried and their effect on particle size and entrapment efficiency was studied. Freeze-dried SLN further characterized using SEM, DSC and PXRD analysis revealed complete entrapment of the drug and amorphous nature of the SLN. In vitro pH release studies in 0.1 N HCl and 6.8 pH buffer demonstrated 84 and 80% release at the end of 12th h. The apparent permeability of the SLN across rat intestine was found to be 24 × 10−6 at 37 °C at the end of 30 min while at 4 °C the same was found to be 5.6 × 10−6 prompting involvement of endocytic processes in the uptake of SLN. Accelerated stability studies revealed no prominent changes upon storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is seen mostly that the in vitro data do not co-relate with those obtained in vivo and the main reason for this happens to be insufficient or poor absorption, rapid metabolism and elimination, e.g. peptidic drugs, distribution of the drug to accompanying tissues (cancer drugs), low aqueous solubility of drugs, high fluctuation in plasma levels of drug which is due to unpredictable bioavailability after peroral administration, and effect of presence of food on plasma levels.
A promising strategy to overcome the aforementioned problems encompasses development of suitable drug carrier systems with potential of releasing the active compound according to the specific requirements of the undergoing therapy. Solid lipid nanoparticles (SLN) not only combine the advantages of colloidal drug carrier systems such as liposomes, polymeric nanoparticles and emulsions but also avoid drawbacks associated with these systems (Chalikwar et al. 2012).
Darunavir, a non-peptidic protease inhibitor, suffers from poor oral bioavailability (37%) as it acts as a substrate for polyglycoprotein (PgP) which causes efflux of the absorbed drug back into the intestinal lumen and also a substrate for cyp3A metabolism (Vermeir et al. 2009). The bioavailability of darunavir can be increased to 82% by co-administering ritonavir, which is a potent cyp3A inhibitor.
Present work attempts to improve bioavailability of darunavir by formulation as lipid nanoparticulates, as these have been reported to improve oral bioavailability of drugs prone to PgP efflux and CYP-mediated first-pass metabolism (Aji Alex et al. 2011). Darunavir degrades at a temperature above its melting point (74 °C) which happens to be a big hurdle in preparation of darunavir SLN by hot emulsification method. To circumvent this, a lipid mixture, melting at temperature less than that of darunavir’s melting point, was used to formulate the SLN. It is believed that the SLN would be taken up by the lymphatic system owing to the lipid carrier and the lipid matrix and bypass the hepatic metabolism and also reduce the PgP efflux (Aji Alex et al. 2011). The novelty of the work lies in successful preparation and characterization of a non-lipidic, temperature degradable anti-HIV drug into a SLN carrier and demonstration of improved permeability of the same.
Materials and method
Materials
Darunavir and glyceryl caprylate were received as a kind gift from Lupin Research Park, Pune and all other chemicals were procured from the local sources.
Methods
Selection of lipid
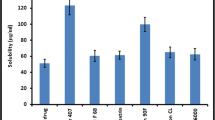
Selection of lipid for preparation of SLN was done on the basis of maximum solubility of drug in lipid. The solubility of darunavir was evaluated in various lipids such as glyceryl monostearate, Compritol ATO 88, Gelucire 43/01, Precirol ATO5, Glyceryl caprylate. Darunavir (50 mg) was weighed accurately and transferred to 50 mg of melted lipid (melting point corresponding to respective lipids) with continuous stirring. Further incremental amount of lipid was added in portions under continuous stirring and heating till a clear solution was formed. The total amount of lipid added to get a clear solution was recorded.
Selection of the surfactant system
The surfactant system was chosen depending upon the average diameter of the particle produced and polydispersity index (PDI) of the resultant SLN dispersion by the surfactant system. Different combinations of lipid- and water-soluble surfactants were employed (Table 1).
Briefly, lipid was taken in a ratio of 5 times that of darunavir, melted at 55 °C and lipid surfactant (2%) was added to this melt, aqueous phase was prepared by dissolving water-soluble surfactant (2%) into distilled water. The two phases were mixed at the same temperature followed by stirring under an over-head stirrer at 15,000 rpm for 1 min to obtain a uniform emulsion, which was further passed through a high-pressure homogenizer (HPH) keeping pressure at 500 bars. The resultant SLN was evaluated for PS and PDI.
Experimental design
A Box–Behnken Design containing 15 experimental runs to evaluate three variables, viz., drug to lipid ratio, concentration of lipid phase surfactant and number of homogenization cycles at 3 levels was employed to determine their effect on two responses, i.e. entrapment efficiency (EE) and particle size (PS) and their interaction therein. The layout of the experimental design and factor coding is shown in Table 2.
The low, medium and high levels of lipid were (1.5, 2.5, 3.5 g), Span 80 was (1, 2, 3%) and homogenization cycles was (1, 3, 5), respectively.
Preparation of SLN
The SLNs were prepared using HPH, as described in selection of surfactant system section. The drug was dissolved in 1 ml of GC, heated to temperature of lipid phase and then added to the lipid phase.
Determination of PS
The PS analysis of the prepared Darunavir SLN dispersion was performed using Malvern zetasizer ZS 90 (Malvern Instruments, Worcestershire, UK). The mean diameter and the poly dispersity index of each batch were recorded.
Determination of EE
Darunavir SLN dispersion was subjected to centrifugation at 20,000 rpm and the pellet of settled SLN was separated from supernatant. The pellet was analysed for the drug content spectrophotometrically at λ 262 nm using chloroform as a solvent; similarly the supernatant was analysed for unentrapped darunavir spectrophotometrically at λ 267 using methanol as a solvent. EE was calculated according to the following equation:
Freeze drying of SLN
The optimized nanosuspension was mixed with various matrix formers such as mannitol, sucrose, microcrystalline cellulose and aerosil in concentrations 50, 100 and 200% w/w to drug (Table 3) and subjected to deep freezing near −20 °C temperature for a period of 24 h. The frozen nanoparticulate dispersion was subjected to lyophilization at room temperature and 0.002 mbar vacuum using Labconco freezone 2.5 lyophilizer (USA).
Evaluation of freeze-dried SLN
PS measurement
The freeze-dried SLNs were suspended in double-distilled water for the PS analysis, which was determined as discussed in determination of PS section.
Drug content
For determining the drug content, 100 mg of freeze-dried SLN was weighed accurately and transferred to a 50-ml conical flask, followed by addition of 10 ml of chloroform and sonication for 10 min. The following solution was filtered and analysed spectrophotometrically at λ 262 nm for drug content. The %EE and %drug loading were determined using the following formulae, respectively.
Surface morphology by surface electron microscopy (SEM)
The freeze-dried SLNs were sputtered with platinum in an ion sputter for 300 s. Images were collected at an acceleration voltage of 15 kV using a back-scattered electron detector on Joel JSM 6360 SEM, USA. Analysis was performed at 25 ± 2 °C.
Zeta potential (ZP) measurement
Zeta potential was determined by measuring the electrophoretic mobility using Malvern Zetasizer Nano ZS 90 (Malvern Instruments, UK). The field strength applied was 20 V cm−1. Prior to the measurement, all samples were diluted in distilled water.
Differential scanning calorimetry (DSC)
Differential scanning calorimetry thermograms for darunavir SLN, bulk darunavir, bulk lipid and physical mixture of lipid and darunavir were generated using DSC823 Mettler Toledo, Mettler Ltd. About 10 mg of sample was weighed and transferred into aluminium pan which was further crimp sealed. The pans were subjected to heating, using an empty pan as reference; over a temperature range of 30 to 300 °C with heating rate of 10 °C per min. Inert atmosphere was provided by purging nitrogen gas flowing at a rate of 40 ml/min.
X-ray diffractometry (XRD)
X-ray scattering measurements were carried out using X-ray diffractometer (PW 3710, Philips Ltd.). A Cu–Ka radiation source was used with the scanning rate (2 h/min) of 5 °C per min. X-ray diffraction measurements were carried out on darunavir SLN, bulk darunavir, bulk lipid and physical mixture of lipid and darunavir.
Effect of pH on in vitro release of darunavir
The effect of pH on the release array of the drug from SLN was evaluated by performing dissolution studies separately in 0.1 N HCl and phosphate buffer with 6.8 pH for 12 h using USP dissolution apparatus (Type II) at 37 ± 2 °C and 50 rpm. The SLN was filled in HPMC capsule and the same was used in dissolution vessel. Aliquots were withdrawn at 1, 2, 3, 4, 6, 8, 10 and 12 h intervals using a 0.2-µm filter (PS of SLN ranged 266–274 nm) and analysed spectrophotometrically at 264 nm to determine the drug content. The kinetics of drug release was studied using PCP disso software.
Ex vivo permeability study
Permeability of the prepared SLN across rat intestine was evaluated using everted rat intestine model (Zhang et al. 2012). One end of the isolated intestine everted using glass rod was clamped and secured with a silk suture, while from the other open end 1 ml of phosphate buffer, pH 6.8, was filled using a syringe. The proximal end was then carefully secured using silk suture and the resultant sac was incubated at 37 °C in dispersion of bulk darunavir (effective concentration of 5 μg/ml) and at 37 and 4 °C in dispersion of darunavir SLN (effective concentration equivalent to 5 μg/ml) for 0.5 h and the fluid in the lumen was analysed for drug content spectrophotometrically at 267 nm.
Accelerated stability studies
The freeze-dried SLNs were stored in capped glass vials at 40 ± 2 °C/75 ± 5% RH for a period of 90 days. Samples were withdrawn at the end of 0, 30, 60 and 90 days to evaluate the PS, EE, zeta potential and drug release as described before.
Results
Selection of lipid
None of the drug lipid combinations displayed clarity despite increasing the amount of lipid up to 500 mg whereas the solubility of the drug in glyceryl caprylate (GC) was found to be 500 mg/ml. The attempt to make a melt dispersion of darunavir in GMS also failed because of degradation beyond melting point (74 °C); hence, a solution of darunavir in GC was prepared (500 mg/ml) and was added to molten GMS maintained at melting point 65 °C to yield clear solution.
Selection of the surfactant system
Table 1 represents findings from different amalgamations of lipid- and water-based surfactant. Tween and Span combination seemed to provide nanoparticles with adequate size and PDI.
Experimental designs
The experiments were designed to study the effect of three independent variables, namely lipid and surfactant concentration and number of homogenization cycle at three levels on response variable PS and percentage entrapment. The batches of Box–Behnken design are represented in Table 2.
Preparation of SLN
SLNs were prepared using HPH as described in the selection of surfactant system section.
Determination of PS
The range of PS distribution was found to be 181–276 nm. The PS of each batch is summarized in Table 2.
Determination of EE
The outcomes of the EE determination are summarized in Table 2. The range of the percentage entrapment was found to be 2–66%. The low EE can be attributed to the nature of the drug (logP 1.76) and its insolubility in GMS.
Optimization data analysis
The formulations prepared as per the experimental design were evaluated and the analysis of experimental results was done using the Stat-Ease Design Expert. The ANOVA, P value and model F value for PS and percent drug entrapment were obtained (Table 4).
F value for both models was found to be high which indicated that the models were significant. P value less than 0.05 indicated that the model terms were significant. High R 2 values indicated good agreement between formulation variables and response parameters.
The statistical model generated for PS was:
The statistical model generated for EE was:
The solution provided by the Design Expert software was, lipid concentration (1.5 g), homogenization cycle (1) and Span (1%), the same had a desirability value of 0.946 towards obtaining optimum parameters for the preparation of SLN. To prove the reliability of the statistics, verification run was carried out further. The optimized formulation had an average PS of 210 nm and EE of 74.23% and in response to the predicted values of 204.5 nm and 71.84% by the software. The percentage error was +3.32 and +2.68% for PS and percentage entrapment, respectively.
Freeze drying of SLN
To facilitate the freeze drying and obtain free-flowing powder without significant increment in PS various matrix formers were used in different concentration as tabulated in Table 3.
Formulations 11 and 12 were obtained with non-sticky powder. Formulation 11 was selected as final formulation as it employed lower amount of matrix former.
Evaluation of freeze-dried SLN
PS measurement
The freeze-dried darunavir SLNs were subjected to PS measurement. An increase in the PS (270 nm) as compared to the size prior to freeze drying (210 nm, D90: 204 nm) was noted.
Drug content
Post-freeze drying, the content of the darunavir in the SLN was decreased from 74.23 to 69.8%. In addition, post-freeze drying the % loading efficiency was found to be 9.37%.
Surface morphology
Surface electron microscopy images revealed presence of smooth, spherical morphology of the SLN (Fig. 1).
Zeta potential measurement
The zeta potential of the freeze-dried SLN was found to be −22 ± 2 mv (n = 3, ±SD), which is desired as negative charge particles are favoured for lymphatic uptake.
Differential scanning calorimeter (DSC)
Differential scanning calorimeter thermograms for darunavir, bulk GMS, physical mixture of darunavir and GMS and Darunavir SLN are represented in Fig. 2.
X-ray diffractometry (XRD)
X-ray diffractograms for bulk darunavir, bulk GMS and darunavir SLN are represented in Fig. 3.
Effect of pH on in vitro release of drug
Darunavir SLN was subjected to dissolution study in differential media, i.e. simulated gastric fluid SGF (0.1 N HCl) and simulated intestinal fluid (SIF) (pH 6.8), both drug release profiles (Fig. 4) were sustained till 12 h (84 and 80% release, respectively). The release of drug from SLN in 0.1 N HCl followed Korsmeyer–Peppas model with r 2 = 0.9816 and n value = 0.851 while the release in 6.8 pH buffer followed zero-order release with r 2 = 0.9759. The slight higher release of darunavir in SGF can be attributed to higher solubility of the same in acidic media.
Ex vivo permeability study
At the end of 30 min, the permeability of bulk darunavir in everted rat intestine model was found to be 2.1 × 10−6 cm/s at 37 °C and 1.9 × 10−6 cm/s at 4 °C while the apparent permeability of the SLN was found to be 24 × 10−6 cm/s at 37 °C and 5.6 × 10−6 cm/s at 4 °C.
Accelerated stability studies
Stability estimation of the freeze-dried SLN was done on the basis of PS, EE and zeta potential. Results showed no significant changes in any of the assessed parameters. The findings of accelerated stability study are represented in Table 5.
Discussion
Selection of lipid
Glyceryl caprylate, chemically glyceryl mono–di-caprylate, is known for its high solubilization capacity owing to its low molecular volume and natural surfactant enhancer activity. The presence of hydroxyl group also plays an important role in solubility of drugs like darunavir in GC (Prajapati et al. 2012). GC, here was used as a co-solubilizer to enhance the solubility in the GMS medium.
Selection of the surfactant system
SLN containing sodium lauryl sulphate (SLS) produced substantially smaller PS, but owing to the toxicity profile (Rowe et al. 2009), high polydispersity index and associated instability of the suspending property, it was eliminated as a choice. Soya lecithin and other combinations of water-soluble surfactants produced particles with high diameter with a high PDI value. The combination of Span 80 and Tween 80 resulted in particles of diameter 346 nm and PDI of 0.280 and hence this system was chosen to carry the study further. Tween as a long-chain surfactant provides aqueous phase stability to the emulsions formed while Span provides the necessary stabilization for the lipidic phase into the continuous aqueous phase thus leading to the formation of an emulsion system of which low PS and PDI is a functional characteristic.
Experimental designs
A three-factor, three-level design would require a total of 27 experimental runs without any repetitions and a total of 30 runs with 3 repetitions (Solanki et al. 2007). A Box–Behnken experimental design reduces the number of experiments to 15, hence was found convenient and appropriate for the study.
Preparation of SLN
It was believed that the aqueous phase would emulsify the lipid phase containing solubilized drug when both phases mixed under stirring and the so-formed pre-emulsions would further be reduced to nanoemulsions under pressure provided by HPH. The resultant when cooled would form SLN incorporated with drug.
Determination of PS
The decrease in the PS can be attributed to the breaking of larger droplets into smaller ones under pressure provided by HPH along with the HLB provided by the Tween and Span 80 surfactant system.
Determination of EE
As the amount of lipid increases the ratio of GC in which drug is dissolved lower in comparison to GMS leading to expulsion of drug, also the magnitude of the pressure from HPH surfactant system lowering PS causes migration of the drug from the lipid system.
Optimization data analysis
The model indicates that lipid at higher concentration contributes in building the PS which may be due to increasing viscosity of the system owing to the fact that increased lipid concentration span had a limited effect of reducing the PS, (Pachuau and Mazumder 2009; Gadhiri et al. 2012; Huang et al. 2008) whereas number of homogenization cycle had most dominant effect which is due to the increased shear provided to break the globules. The interaction term AB, i.e. when lipid and Span are increased simultaneously, caused reduction in the PS which can be attributed to smaller hydrophilic head of span (Gadhiri et al. 2012). The interaction term AC, i.e. when lipid and homogenization cycles were increased together, caused moderate decrease in the PS which may be due to the presence of lipid. The interaction term BC i.e. when Span and homogenization cycles were increased, caused pronounced decrease in the PS which is because both the factors are responsible for reduction of PS. The interaction terms A 2 and B 2 which are higher order terms increase PS, as lipid builds larger particles but when only Span is increased beyond a limit, its lipoid nature also contributes to increasing PS. Increasing the homogenization cycles by any magnitude always had the same effect of reducing the PS. Similar effect was shown by the response surface plots generated (Figs. 5, 6, 7).
The model indicates that the lipid contributes to the decrease in the EE which is contrary to the general observation, this can be explained with the understanding of lowering of GC:GMS ratio with increase in GMS content with respect to ratio of GC which is fixed and increase in the GMS causes lower availability of GC to keep drug in solution leading to its expulsion and reduced entrapment. Hence, there arises a need to optimize the lipid content. The role of the surfactant concentration and homogenization cycle is seen to contribute to reduction of drug entrapment which is because of lower PS of globule leading to enhanced area for migration of drug to aqueous phase. However, the interaction between lipid and surfactant (AB) led to increase in the EE due to contribution of Span in lipid phase. The interaction between lipid and homogenization cycle (AC) shows a minor decrease in the entrapment which is due to the counteraction of the effect of homogenization by increased presence of lipid. The higher order terms, i.e. A 2, B 2 and C 2 indicate increase in the EE. Similar effects were shown by the response surface plots generated (Figs. 8, 9).
Freeze drying of SLN
Sucrose and mannitol provided cryoprotection in concentration ranges of 100 and 200% but the lyophilized powder retained stickiness immediately after removal of samples which may be due to hygroscopic nature of the sugars. Thus, it was eliminated as a choice.
Evaluation of freeze-dried SLN
PS measurement
The increase in the PS post-freeze drying could be because of the fusion of particles and/or polymorphic transition of the lipid (transformation of higher energy α and β′ modification to the lower energy β modification) in the process of being lyophilized (Liu et al. 2014).
Drug content
The reason for the decrease of darunavir content in nanoparticulates could be polymorphic transition of the lipid leading to expulsion/leaking/leaching of drug from the SLN during the progression of freeze drying (Liu et al. 2014).
Surface morphology
The spherical and smooth nature of the particle is often favoured for the uptake through the cells of the lymphatic tissue owing to the ease of uptake of the spherical particles as compared to the uneven and disfigured particles (Champion et al. 2007).
Zeta potential measurement
The negative charge on the surface of the nanoparticle is believed to facilitate uptake from the intestine by the Payers patch, leading to the lymphatic circulation, also it is believed to prevent entangling of the nanoparticles in the negatively charged mucous owing to the repulsion of like charges (Kovačević et al. 2014).
Differential scanning calorimeter (DSC)
A sharp peak at 85 °C for darunavir (A) followed by further peaks representing the polymorphic behaviour, peak at 60 °C representing the melting of GMS (B) and two individual peaks at 60 and 85 °C for physical mixture of darunavir and GMS indicating absence of any interaction between the two were seen. The thermogram for darunavir SLN showed a single broad peak at 55 °C indicating molecular mixing of the amorphous drug with the lipid GMS. The decrease in the melting temperature can be explained to be a result of conversion of GMS into stable β form during heating and cooling operations in SLN preparation (Souto et al. 2008).
X-ray diffractometry (XRD)
X-ray diffractograms for darunavir and GMS exhibited sharp crystalline peaks which are absent in the diffractogram of darunavir SLN, indicating complete molecular level miscibility of the drug in the GMS and presence of the drug in amorphous form (Ravi et al. 2014).
Effect of pH on in vitro release of drug
The reason for the comparatively higher release of darunavir in 0.1 N HCl can be attributed to the increased solubility of darunavir in acidic medium. Figure 4 represents the release profile of darunavir in 0.1 N HCl and 6.8 pH. In HCl, the SLN followed Korsmeyer–Peppas model with an ‘n’ value of 0.851 meaning an anomalous release mechanism combining diffusion and erosion while in 6.8 pH the release was of zero order.
Ex vivo permeability study
At 4 °C the endocytic processes are diminished which was the reason for the decreased permeability of the SLN in the experimental sample kept at 4 °C thus confirmed involvement of endocytosis in uptake of darunavir SLN was established (Ravi et al. 2014). The increased permeability of the SLN over bulk drug can be attributed to the lipid matrix in which drug is entrapped as lipid being favoured for uptake by the M cells in the intestinal epithelium.
Accelerated stability studies
It is reported that, upon ageing, drug expulsion from solid lipid matrix (due to crystallization of lipid) leads to reduction in EE which being the reason for the decrease %EE of SLN upon stability storage (Müller et al. 2011).
Conclusion
Darunavir SLN prepared by HPH method using Box–Behnken design was found to have PS-270 nm, %EE 69.8% and smooth spherical surface. Ex vivo permeability studies demonstrated endocytic uptake of the SLN thus establishing the hypothesis that lymphatic transport can be followed by the prepared SLN to increase the systemic availability and the demonstration of the same is under experimentation.
Thus, it can be concluded that HPH method can be successfully employed to prepare darunavir SLN which is believed to have potential to increase the systemic availability of the drug owing to endocytic uptake and lymphatic transport therein.
Future perspective
The authors believe that the future of the aforementioned research work shall be evaluation of the impact of the surface charge on the lymphatic uptake of SLN. The research is believed to act as a platform technology for delivery of drug molecules exhibiting low bioavailability owing to extensive metabolism, especially, anti-retrovirals, proteins and peptides.
References
Aji Alex MR, Chacko AJ, Jose S et al (2011) Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci 42(1–2):11–18
Chalikwar SS, Belgamwar VS, Talele VR et al (2012) Formulation and evaluation of nimodipine loaded solid lipid nanoparticle delivered via lymphatic transport system. Colloid Surf B. 97:109–116
Champion JA, Katare YK, Mitragotri S (2007) Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release 121(1–2):3–9
Gadhiri M, Shohreh F, Vatanara A et al (2012) Loading hydrophilic drug in solid lipid media as nanoparticles: statistical modeling of EE and PS. Int J Pharm 424(1–2):128–137
Huang LF, Du YZ, Yuan H et al (2008) Solid lipid nanoparticles prepared by solvent diffusion method in a nanoreactor system. Colloid Surf B 61(2):132–137
Kovačević AB, Muller RH, Savic SD et al (2014) Solid lipid nanoparticles (SLN) stabilized with polyhydroxy surfactants: preparation, characterization and physical stability investigation. Colloid Surf A. 444:15–25
Liu J, Jiang W, Feng-sheng L et al (2014) Effect of drying on ps and sensitivities of nano hexahydro-1,3,5-trinitro-1,3,5-triazine. Def Technol 10(1):9–16
Müller R, Shegokar R, Keck C (2011) 20 years of lipid nanoparticles (SLN & NLC): present state of development & industrial applications. Curr Drug Discov Technol 8(3):207–227
Pachuau L, Mazumder B (2009) A study on the effects of different surfactants on Ethylcellulose microspheres. Int J PharmTech Res 1(4):966–971
Prajapati H, Dalrymple D, Serajuddin AT (2012) A comparative evaluation of mono-, di- and triglyceride of medium chain fatty acids by lipid/surfactant/water phase diagram, solubility determination and dispersion testing for application in pharmaceutical dosage form development. Pharm Res 29(1):285–305
Ravi PR, Kathuria H, Aditya N, Malekar S, Vats R (2014) Lipid nanoparticles for oral delivery of raloxifene: optimization, stability, in vivo evaluation and uptake mechanism. Eur J Pharm Biopharm 87(1):114–124
Rowe RC, Sheskey PJ, Quinn M (2009) Hand book of pharmaceutical excipients, vol 6. Pharmaceutical Press, London, UK, p 653
Solanki AB, Parikh JR, Parikh RH (2007) Formulation and optimization of piroxicam proniosomes by 3-factor, 3-level box-Behnken design. AAPS Pharm Sci Tech 8(4):1–7
Souto EB, Muller RH, Runge SA et al (2008) Cyclosporine-loaded solid lipid nanoparticles (SLN): drug-lipid physicochemical interactions and characterization of drug incorporation. Eur J Pharm Biopharm 68(3):535–544
Vermeir M, Lachau-Durand S, Mannens G et al (2009) Absorption, metabolism, and excretion of darunavir, a new protease inhibitor, administered alone and with low-dose ritonavir in healthy subjects. Drug Metab Dispos 37:809–820
Zhang Z, Gao F, Bu H, Xiao J et al (2012) Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: in vitro characteristics and absorption mechanism in rats. Nanomedicine. 8:740–747
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bhalekar, M., Upadhaya, P. & Madgulkar, A. Formulation and characterization of solid lipid nanoparticles for an anti-retroviral drug darunavir. Appl Nanosci 7, 47–57 (2017). https://doi.org/10.1007/s13204-017-0547-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-017-0547-1