Abstract
Until recently the drive to discover and utilize renewable feedstocks for the production of energy and for the manufacture of materials was exceptionally strong. Now, however, because of the realisation that nonrenewable (e.g. gas and oil) reserves are still superabundant, a different emphasis is appearing. This involves utilizing both nonrenewable and renewable feedstocks in an environmentally responsible manner. One important recent development involves the drive to utilize feedstocks, such as pyrolysis oil, microalgae and general bio-waste, like sawdust and other nonedible products from lignocellulose. Another is the aim to ensure that CO2 can be converted to fuels or useful materials, thereby diminishing its concentration in the atmosphere. This paper focuses on these themes; but it also addresses other important specific questions. Among these, the following are of particular interest: (i) How may catalytic cracking be made more environmentally acceptable? (ii) The emergence of single atom catalysts as means of effecting important chemical reactions.
Similar content being viewed by others
Introduction
For a variety of reasons, the title of this article is significantly different, and so is its content, from the lecture I (JMT) gave on “Some of Tomorrow’s Catalysts: Actual and Desired” at the KOPRC Forum in August, 2015. In the relatively short interval that has elapsed since that Forum, many significant new developments have occurred in the fields circumscribed by the title of this article. Also, I have been the author or co-author of other related articles, which have appeared, or are shortly to appear, that address many of the questions raised by this broad field. Moreover, even before presenting my KOPRC talk at Oxford, I had reviewed in detail the many facets that arise in considering how humankind is going to cope with powering the planet in an environmentally responsible manner in the next 50 years and beyond—see, for example, Refs. [1–5]. See also the definitive review by Centi and co-workers [6] published in 2015, and references therein. Hence, the situation pertaining to all of the topics relevant to those touched upon or implied by the title of this paper is rapidly changing. For example, I did not know until late October 2015 that there is now perceived to be enough natural gas available in the world to serve the needs of the planet for an estimated 230 years! So the frequently repeated mantra [7] that it is necessary to seek more renewable sources of energy is simply not true. For example, as recently as 9 January 2016, it was announced [8] that Australia is increasing natural gas production by roughly 150 % over the next 4 years.
In addition to these macro developments, progress in the evolution of new catalysts, especially for the processing of CO2, continue to be reported. Furthermore, the agreement reached in the UN meeting in Paris, December 2015, calls for further action to be taken, not only to stabilize but to decrease the amount of anthropogenically produced CO2 in the Earth’s atmosphere if the target of keeping the temperature rise of the Earth to less than 2 °C by 2030 is to be met.
In predicting and pontificating about what catalysts are likely to be needed and used to fulfil the desiderata implied by the title of this paper, it is salutary to recall that numerous, well-intentioned predictions—often made by experts of unimpeachable credentials—tend to fall short of realisation. As in my Oxford lecture, I recall here that the Commission set up by President Roosevelt to advise him on scientific and technological developments in the foreseeable future, missed many seemingly obvious developments when they reported in 1937. They made no mention of the future, expected use of antibiotics, despite the fact that Alexander Fleming had discovered penicillin in 1928. They also did not find it pertinent to mention that, in future, devices such as the fax machine (invented in the early 1840s) or the fuel cell (also first described and tested in the 1840s) would ultimately be in popular use.
Given the fact that there is still an abundance (almost a superabundance) of fossil-based feedstocks, and also that there is public clamour for the operations of a civilized life to generate less CO2, all kinds of socio-political, as well as technological, initiatives are constantly being pursued.
What is absolutely necessary is that more efficient ways are needed of utilizing nonrenewable feedstocks. To give an interesting recent example, aircraft manufacturers are “rethinking the airplane for climate sake”. (This statement figured as the title of an article in the International New York Times in January 2016). In essence, the concept called distributed propulsion is one of several being studied by the NASA Armstrong Flight Research Center in California, to develop technologies that could lead to completely new and far less polluting aircraft designs. For example, future planes may be powered by batteries or hybrid gas-electric systems, and have lighter wings that can quickly change shape so as to handle better the stresses brought on by turbulent air.
Converting carbon dioxide to fuel and other products
It is well known that the French worker, Sabatier, over a Century ago, demonstrated how methane could be produced synthetically by passing a mixture of CO2 and H2 over a supported Ni catalyst:
Previously, when it was felt that natural gas could be in short supply in various parts of the world, the Sabatier reaction could be used to produce synthetic natural gas (SNG), for transport and other purposes. Nowadays, it is not the thermally activated Sabatier reaction that is attracting the attention of those chemists devoted to the diminution of anthropogenic CO2 but its photocatalytic equivalent. At present, there is no proven, highly active and desirable photocatalyst that has been discovered to convert CO2 to CH4 in a sustained manner and with high efficiency. The quest for such a photocatalyst, discussed by workers such as Corma et al. [9] and Ozin et al. [10] is likely to last for a long time.
For several decades CO2 has been used as a feedstock to produce salicylic acid (a precursor in the manufacture of aspirin), and urea, the most compact form of soil fertiliser. But these industrial processes, although they utilize CO2 as a feedstock, are far from being carbon neutral. It is gratifying to note, however, that many of the polymeric products previously synthesized from nonrenewable sources can now be readily prepared in an entirely sustainable manner. The case of polyurethane is a good example. Langanke et al. [11] have shown how CO2 can be used as feedstocks in copolymerizations using epoxides—see Fig. 1. In fact, the Bayer material science (BMS) organisation now produces this industrial synthesis on the scale of 103 ton per annum.
a Copolymerization of epoxides and CO2 to alternating poly-carbonates (top; * = end group) and polyethercarbonates (bottom). b Tailored polyethercarbonate polyols obtained from propylene oxide and CO2 using zinc hexacyanocobaltate (DMC) as a catalyst and a multi- functional alcohol as a starter (After Langanke et al. [11])
What is glaringly obvious is that the amount of CO2 liberated into the atmosphere as a result of human activity (some 50 gigatonnes per annum) far exceeds the amount of CO2 currently used as a feedstock for the production of useful materials. The latter amounts, at present, languish in the megaton range per annum.
A constructive development is the use of CO2 (sunlight and water) as a feedstock for the growth of algae using genetically enhanced cyanobacterias to generate ethanol and O2—see Fig. 2. This work is carried out by the Algenol Biofuels Company, which, in its facility in Florida, utilizes CO2 gas liberated from an industrial plant. At present, this Company produces 8000 gallons of ethanol per acre per year, and they quote a solar energy conversion of 2–3 %. When their plant grows to occupy 2000 acres (at the seashore [12]), the Algenol Company will produce ca. 14 x 106 gallons of ethanol per annum. An important advantage possessed by producing ethanol from algae rather than corn is that no land is used up that could otherwise have been used to produce food. The algae bio-photoreactors all operate at the seashore.
Important as this development is, its merits need to be carefully stated:
-
it converts anthropogenically produced CO2 to generate ethanol;
-
this ethanol can be readily catalytically dehydrated to yield ethylene using a number of single-site heterogeneous catalysts, such as DAF-4, SAPO-34 and others, as described elsewhere [13] by one of us (JMT);
-
if the final use of the ethanol is as a fuel (blended into gasoline) or as a source of polyethylene, its ultimate fate is to generate more CO2, which, however, can then be used as feedstock in subsequent algal-based solar-driven production of more ethanol.
We shall return below to the important part microalgae are likely to play in future production of “green” hydrocarbons (i.e. sulphur-free) and diesel fuels. Other biochemically oriented methods of consuming CO2 as a feedstock are being explored by the German investigator, Erb [14]. Such work involves the creation of customized, CO2-fixing biochemical pathways to produce biomass and fine chemicals from atmospheric CO2.
In Erb’s work, Rubisco chemistry figures eminently: Rubisco is d-ribulose-1, 5-bisphosphate carboxylase/oxyglucose. This is a member of the carboxylase family of enzymes—see Thomas and Harris [5] for more details. Rubisco chemistry has also influenced the recent, ingenious work of Kanan et al. [15, 16]. These workers set out to emulate nature’s strategy for C–C bond formation, which is to deprotonate C–H bonds to form carbanions and then to trap these intermediates with CO2 to form C–CO2 moieties. They use a purely inorganic approach, which has the advantage of circumventing the need to select the appropriate, robust enzyme co-factor, and some other complications associated with the approach of the synthetic biologist.
What is commendable about what Kanan et al. [15] accomplish is their ability to synthesize ethylene glycol and ethanol using only CO2 and H2. Their work leads to the ready synthesis of polyethylene furandicarboxylate—also designated polyethylene furanoate, PEF—which is a viable substitute for polyethylene terephthalate (PET) [17], and is used extensively as a container material for portable water and mineral drinks.
The H2 required for the preparation of ethylene glycol—a powerful chemical building block for other materials—may be produced by either wind-powered or solar-powered water-splitting, using either wind or photovoltaic power for electrolysis. Kanan [15] estimates that by replacing the entire 15 M tonne year−1 PET market with PEF, 20–35 M tonne year−1 of CO2 would be saved from liberation to the environment.
It is relevant at this junction to note that Freund [18] and colleagues in Berlin have constructed a method of attaching a neutral CO2 molecule to a radical ion of CO2, thus forming a \(( {\text{CO}}_{ 2} )_{ 2}^{ - }\) species, which may then be transformed into an oxalate species, whereby a C–C bond is formed. These oxalate species may then be further catalytically transformed with water or ammonia into useful fuels and other products.
Some other relevant aspects of converting CO2 to fuel
It has been estimated (by environmentalists and city planners) that about 40 % of all fossil fuels are utilized in heating homes and other buildings. As a consequence, revolutionary methods are now being invoked to seek alternative methods of achieving the minimum amount of nonrenewable feedstock for this purpose. It is, therefore, relevant to note that an apartment block in Hamburg (Germany) has been built that uses microalgae placed within the façade to generate heat and biomass. This has been done by engineers [19] who are engaged in the establishment of zero—or even surplus—energy buildings as part of the goal to achieve sustainable architecture.
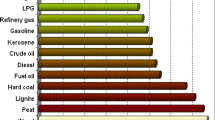
The solar fuel network is an international community of scientists dedicated to the idea of establishing a situation in which solar energy is harnessed for the production of fuels that can ultimately replace those derived from nonrenewable sources. Bearing in mind the scale of the operations involved when all the fuels and other products of the catalytic cracking of petroleum are considered—see Figs. 3 and 4 for some relevant quantitative facts—this is indeed a tall order, as has been pointed out previously [20]. Faced with the magnitude of this task, some scientists declare that there is a long road still to be travelled before we see the onset of widespread use of solar fuels.
On the other hand, as pointed out by Jacobson et al. [21, 22], civil engineers in Stanford University and the Nobel prizewinning Paul Krugman, in a recent (February 2016) article in the International New York Times, “wind, sun and tide”, are already making a significant contribution to civilized life. In the main, these three sources of power are used to convert sunlight to an abundant generation of H2. It is also relevant to mention that numerous concentrated solar power plants of 200 and 300 MW are also capable of providing the electricity to electrolyze water for the generation of H2. Krugman’s article quoted a recent report by the investment firm Lazard that the cost of electricity generation using wind power fell 61 % from 2009 to 2013, while the cost of solar power fell 82 % in the same period. This trend is continuing.
But whilst there are increasingly more viable methods of generating relatively cheap H2—which can sustain the hydrogen economy, and bolster the use of fuel cells (burning H2) for transport, hospitals, shopping malls and the like—the next goal is to achieve highly efficient photocatalytic conversion of CO2 to CH4. In other words, it is the photochemical Sabatier reaction that needs to be conquered. (It must not be forgotten that as much energy from the sun reaches the Earth’s surface in a few hours as the world currently consumes (largely as fossil fuel) in 1 year.)
To date, some significant progress has been made. For example, Sastre et al. [9] have made a worthwhile attempt to achieve complete photocatalytic reduction of CO2 to methane by H2 under solar light irradiation (Fig. 5). Ozin and co-workers [23, 24] have also made a promising start, but their catalyst (In2O3−x(OH)y) goes only as far as photocatalytically converting CO2 to CO (which is still a worthwhile goal). In a thorough review of the entire question of photocatalytically converting CO2 to CH4, Dhakshinamoorthy et al. [7], focusing on TiO2 as the photocatalyst, concluded that the average productivity of the less-TiO2-based photocatalyst is about 100 μ mol g−1 h−1 of catalyst with sunlight. They emphasized the need to increase this productivity by orders of magnitude. In the main, the solar fuels community has concentrated most of its efforts on improving the efficiency of the hydrogen evolution reaction (HER), by employing solar light to drive a photochemical cell typified by the set up: TiO2−Ti−pn + Si as photocathode. At a symposium organised by J. A. Barber for the Solar Fuels Network (London, July 2015), a protagonist in the field, Professor Harry Gray of Caltech, urged those working on the generation of solar fuels to concentrate more on using CO2 rather than H2O as the material for the production of useful fuels.
A recent report in the Journal of the American Chemical Society towards complete photocatalytic reduction of CO2 to methane by H2 under solar light irradiation (After Sastre et al. [9])
An attractive feature of the photocatalytic conversion of CO2 to CH4 is that if successful on a large scale, the practice of blending H2 with natural gas—which involves careful control of the relative amounts of H2 and natural gas—can be replaced by the much safer and utilitarian blending of sunlight-derived CH4 with the natural gas grid. This strategy, if and when successful, would ultimately stabilize the amount of CO2 in the Earth’s atmosphere (CO2 → CH4 → CO2 → CH4, etc.).
A few further remarks on TiO2 are appropriate here. The high oxidation potential of photogenerated holes makes bulk TiO2 a powerful oxidant for water and organic molecules, a fact that has given rise to many practical applications of solid state photocatalytic activity, such as self-cleaning windows, tiles and cements (by Anpo et al. [25, 26]), anti-bacterial paints, and the purification of water by the solar breakdown of dyestuffs and other organic pollutants.
Recently, one of the present authors (JMT) has been associated with a fundamental study [27] of a single-site Ti(IV)-based photocatalyst, a titanosilicate, known as JDF-L1 [28], with the formula: Na4Ti2Si8O224H2O. The essential features of the active centre in this open-structure solid, a TiO5 arrangement, square pyramid, are shown in Fig. 6, in which there is one Ti=O apical bond and four Ti–O bonds. 4D ultrafast electron microscopy showed [27] that upon photoexcitation, the Ti(IV)=O bond is transformed to a single bond Ti(III)–O− with a consequential dilation of the length of the double bond from 1.7 Å–2.5 Å. This occurs on the femtosecond time scale, and a schematic illustration of this process as well as the ensuing photochemical possibilities are shown in Fig. 7. This illustration depicts the kind of pathways that are followed in the photoreduction of CO2 or H2O by the JDF-L1 catalyst.
5-coordinated titanium at the catalytic active centre of the photocatalytic titanosilicate JDF-L1 (After Yoo et al. [27])
Schematic illustration from a 4D ultrafast electron microscopy study of the titanium active centre of JDF-L1, showing photocatalytic reduction of CO2 and H2O and emphasizing the femtosecond laser induced electron transfer and subsequent Ti=O bond dilation (After Yoo et al. [27])
Very recently, Ozin and co-workers investigated the unique photoactive behaviour of pristine and defected indium oxide surfaces [24]. Their combined theoretical and experimental study provided fundamental insights into excited state properties, as well as an explanation for the experimentally observed enhanced activity of defected indium oxide surfaces for the gas-phase reverse water gas shift reaction, CO2 + H2 + hν → CO + H2O, in the light compared to the dark (Fig. 8). A thorough excited state study of pristine and defected forms of indium oxide (In2O3, In2O3−x, In2O3(OH)y and In2O3−x(OH)y) surfaces was carried out using time dependent density functional theory calculations. The results were supported experimentally by transient absorption spectroscopy and photoconductivity measurements. They found that the surface frustrated Lewis pairs created by a Lewis acidic coordinately unsaturated surface indium site proximal to an oxygen vacancy and a Lewis basic surface hydroxide site in In2O3−x(OH)y become more acidic and basic, and hence more active in the excited state compared to the ground state. They described how this provides a theoretical mechanism responsible for the enhanced activity and reduced activation energy of the photochemical reverse water gas shift reaction observed experimentally for In2O3−x(OH)y compared to the thermochemical reaction. To conclude, they emphasize that such fundamental insight into the role of photoexcited surface frustrated Lewis pairs for catalytic CO2 reduction could lead to improved photocatalysts for solar fuel production.
(Left) Visualization of the main contributing molecular orbitals and transition densities for a S1 (HOMO → LUMO), b S2 (HOMO → LUMO + 1) and c S3 (HOMO → LUMO + 2) excited states of a defected In2O3−x(OH)y surface. (Right) Schematic illustration of the origin of the difference in the experimental activation energy (ΔEa)Expt. for the reverse water gas shift reaction CO2 + H2 → CO + H2O involving the ground state surface frustrated Lewis pair in the dark and excited state surface frustrated Lewis pair in the light. The computational analyses of the ground state and excited state of the In2O3−x(OH)y cluster model showed that the electrons and holes trapped at the Lewis base and Lewis acid sites of the excited state surface frustrated Lewis pair enhance their Lewis basicity and Lewis acidity compared to the ground state frustrated Lewis pair. This increased ‘frustration of charges’ at the surface Lewis pair consequently decreases the activation barrier for the overall CO2 reduction reaction, which is supported by the experimental measurements (After Ghuman et al. [24])
It is relevant to note that very recently a novel approach, spearheaded by Ozin and colleagues in Canada and involving collaborators from other countries, uses visible and near infrared radiation to effect photothermal (not photocatalytic) conversion of CO2 in the presence of H2 to CO (i.e. the reverse water gas shift reaction) [29]. The solids used by the workers consist of Pd nanocrystals supported on nanorods of Nb2O5. Conversion rates as high as 1.8 mmol g−1 h−1 were achieved. Careful experiments disclosed that the photothermal catalysis originates from intra-band and/or inter-band optical excitation and nonradiative relaxation of Pd nanocrystals rather than being driven by UV plasmon excitation of the Pd nanocrystals or by electron-holes pair generation upon absorption of UV photons in the Nb2O5.
Environmentally benign processing of renewable and nonrenewable feedstocks
One of us (JMT) has recently given an account of many of the ways in which the action subsumed by this sub-heading can be fulfilled; and the reader is referred to that article, Ref [5]. There are, however, many more factors to outline in addition to those contained in Ref [5]. Three particular issues are considered here: catalytic cracking; the generation of bio-oil from renewable feedstocks; and the emergence of a new class of heterogeneous catalysts, i.e. those that are composed of supported single atoms.
Catalytic cracking
This is an extremely important applied catalytic activity in that a large majority of the materials used in civilized life are currently the products of the cracking of petroleum—see again Fig. 4. It is unlikely, in the short term, that the catalytic cracking of petroleum will cease because it is well-nigh indispensable throughout the world. What is, however, desirable is that the process can be made more efficient. The yield of desirable products must be significantly increased. Fortunately, significant progress has recently been made in this direction. Before describing it, however, it is relevant to recall the scale of the operation of fluidized catalytic cracking (FCC) of petroleum.
Figure 3 reminds us of the massive scale of the cracking process by considering one single 75,000 barrel-a-day operation. And Fig. 9 illustrates the nature of the catalytically active centre (an (LaOH)2+ entity) inside the cavities of a faujasitic zeolite. In industrial parlance, this is the La-Y ultra-stabilized Brønsted acid zeolite used on a massive scale—it is estimated that 500 K tonnes of the cracking catalyst are ‘consumed’ each year—to break down the hydrocarbons of petroleum into small, more useful products, such as light alkanes and alkenes, as well as octanes and other hydrocarbons of larger molecular weight suitable as the fuel for diesel engines.
That such enormous amounts of the zeolite Y (La3+-exchanged) cracking catalyst are consumed every year arises because the catalyst matrix [consisting of zeolite Y, kaolin (as a filler), and typically, aluminium chlorohydrate (as a binder)] are friable and lack attrition resistance. Another important fact pertaining to faujasitic zeolite cracking catalysts is that they have quite large—but not large enough (see below)—pores (ca. 7.4 Å diameter). Thanks primarily to the work of the Spanish investigator, Garcia-Martinez et al. [30, 31], mesostructural zeolite Y has been prepared and is already now being utilized industrially. Mesoporous zeolites [32] and other so-called hierarchically structured zeolites are synthesized using large micelles made up of controllable diameter that consist of a long-chain surfactant template—see Fig. 10. The essential difference between a conventional zeolite (like Zeolite Y) and a mesostructured one is shown in Fig. 11, which also illustrates the improved pore characteristics of the mesoporous variety. (The Rive Company in the US manufactures the variety of mesoporous zeolites designed by Garcia-Martinez et al.). Additional indications of the improved catalytic performance of the Rive-type of mesoporous cracking catalyst are shown in Fig. 12.
The cumulative pore volumes of a conventional cracking catalyst (blue) and a mesostructured cracking catalyst (red), prepared by Rive (After Speronello et al. [31])
The mesoporous Rive US Y zeolite catalyst shows significantly superior performance compared with the conventional cracking catalyst (After Speronello et al. [31])
The overall message derived from the introduction of mesostructured zeolites—and additional messages are continued in the collection of articles contained in Ref [32]—is that by judicious design of new solid catalysts, more efficient ways can be found for processing nonrenewable feedstocks.
A selection of important manufacturing processes that can be improved by utilizing designed new solid catalysts are given in the review by Harris and Thomas [5] and also in Refs. 1, 2, 13. Both catalytic dehydration reactions and a host of other processes can be facilitated by nanoporous catalysts that contain one or more types of spatially well-isolated active centres epitomized by the bifunctional catalyst shown in Fig. 13.
Sustainable chemistry by upgrading pyrolysis oil
(This section is based largely on Sect. 9.7.1 of the book by Thomas and Thomas [2]).
So-called bio-oil is produced by fast pyrolysis or liquefaction of lignocellulosic biomass. It is an aqueous highly functionalized, but essentially sulphur-free, mixture of light to medium hydrocarbons containing up to 30 % water. Because it is corrosive and possesses rather low ‘heating value’ (<19 M J kg−1), and some other disadvantageous features, it is unattractive for use without prior treatment. If it is subjected to hydrogenation and hydrodeoxygenation (HDO) with cobalt-doped MoS2 catalysts, high-grade transportation fuels may be extracted from it.
An alternative approach based on zeolites involves simultaneously catalysing several reactions, including dehydration, cracking, polymerization, deoxygenation and aromatization at temperatures between 350° and 450°. These conditions serve to convert the oxygenates into aromatic molecules and carbonaceous deposits, while the yield of alkanes does not exceed some 25 %. Very recently, Lercher [33, 34] has shown how widely applicable zeolitic catalyst HZSM-5 with a pore system containing a substantial fraction of Ni metal nanoparticles can quantitatively produce C5–C9 hydrocarbons from paraffins, napthenes and aromatic molecules in a cascade reaction by HDO of n-hexane-extracted crude bio-oil in the presence of substantial concentrations of water under mild reaction conditions (250° and 5 MPa H2).
The components of the n-hexane-extracted bio-oil include mainly C5–C6 substituted furans, ketones and aldehydes derived from the deconstruction of cellulose and hemicellulose, as well as C6–C9 substituted phenols derived from the deconstruction of lignin (Fig. 14a). HDO of such a mixture on Ni/HZSM-5 in a semi-batch reaction for 4 h succeeded in quantitative conversion into the corresponding C5–C9 hydrocarbons, as shown in Fig. 14b.
Upgrading of pyrolysis oil over Ni/H5 M-5 by cascade reactions. GC (gradient correction) analysis of mixtures before and after upgrading: a n-hexane extracted crude bio-oil and b hydrocarbon products (After Zhao and Lercher [33])
Many other studies reporting fast (or slow) pyrolysis of biomass have recently appeared. In one, emanating from the US Department of Agriculture [35], sustainable production of bioenergy and biochar from the straw of high-biomass soybean is described in the context of ‘on-farm’ biorefinery, where food and bioenergy can be sustainably produced. The biochar retains mineral constituents that are beneficial to plants and is deployed for soil remediation.
Catalytic conversion of microalgae into green hydrocarbons and ethanol
As mentioned above, microalgae constitute a very viable source of ‘green’ hydrocarbons. This arises because of their high cellular lipid content and unusually high rate of photosynthetic growth. Algae mass captures about 3–8 % of incident solar energy, in contrast to terrestrial plants, which do so at about 0.5 %. Some microalgae have higher than 60 % oil content by weight of dry biomass, and the average oil content attains approximately 20–50 %. Microalgae grow 12 times as fast and yield 30 times as much triglycerides per unit area compared to conventional oil-producing land plants such as sunflower and rape. Moreover, they do not require arable land—where they would compete with the production of food—and they can utilize waste water, sea water and industrial CO2 (flue gases) to grow as valuable biomass.
It is estimated that there are between 60,000 and 80,000 species of algae; and the number of products that may be derived from them are many and varied (especially from those that are genetically engineered): medicines, pharmaceuticals, foodstuffs, jet fuel, bioethanol, biodiesel and ingredients for several consumer products. In this brief review, we shall focus on but two main products: diesel-range alkanes and bioethanol.
Microalgae to diesel
Currently, three approaches are used for microalgae oil refining. The first involves transesterification of triglycerides and alcohol into fatty acid alkyl esters (FAAEs) and glycerol. The second employs conventional hydrotreating catalysts, such as sulfided NiMo and CoMo for upgrading. The third, which is the one we focus on here, relies on supported noble and base metal catalysts for decarboxylation and decarbonylation of carboxylic acids to alkanes at 300–300 °C, but these catalysts exhibited low activities and selectivities for C15–C18 alkanes when triglycerides were converted.
Recently, a more efficient method has been described by Peng et al. [36]. who used a novel Ni catalyst supported on an acidic zeolite, HBeta. This quantitatively converts crude microalgae oil under mild conditions (260 °C, 40 bar H2) to diesel-range alkanes as high-grade second-generation transportation biofuels. From measurements of product distributions for this transformation, these authors formulated the reaction mechanism given in Fig. 15. The pathway proceeds through an initial metal-catalysed hydrogenation of double bonds in the alkyl chain, followed by hydrogenolyses of the formed saturated triglyceride leading to fatty acid and propane. The subsequent hydrogenation of the carboxylic groups of fatty acid leads to the corresponding aldehyde (rate-determining step), followed by either decarbonylation or hydrogenation. Subsequent acid-catalysed dehydration and metal-catalysed hydrogenation lead to the long-chain n-alkane. Because of an abundance of acidic sites in the zeolite, hydroisomerization and hydrocracking of the alkanes also takes place; and CO may react with H2 to produce methane (by the classic methanation reaction).
Proposed reaction pathway for the transformation of microalgae oil to alkanes over bifunctional Ni/HBeta catalysts (After Peng et al. [36])
The Licella approach
Using catalytic hydrothermal technology, Maschmeyer and colleagues [37] have recently developed (and commercialized) a catalytic hydrothermal reactor which converts feedstocks such as sawdust into bio-crude oil of such good quality—it is essentially free of sulphur—that it can be readily blended with stocks of either diesel, kerosene or gasoline. The first commercial plant, now being completed in Canada, is intended to transform 200,000 tonnes of waste annually into the desired ‘green’ bio-oil. Figure 16 illustrates the overall conversion.
Essence of the Cat-HTR process [37]
This development is of great significance in this environmentally conscious age, especially when one reflects on the fact that only some 18 % of a tree is used in paper production; it therefore means that converting the ‘tree waste’ into bio-oil is a significant step forward.
Single atom heterogeneous catalysts
Great interest is now being given to the increasing variety of this category of catalysts. Short accounts have been given elsewhere—see Thomas and Harris [5], Yang et al. [38], Flytzani-Stephanopoulos [39], Vile et al. [40] and Thomas [41]. A spectacular example of a stable single atom Pd catalyst for selective hydrogenation has recently been reported by Vile et al. [40]. Unlike many other previous workers in this field, they use a structurally adept solid as the support for atomically dispersed Pd, namely, nanoporous carbon nitride, C3N4. The merit of their system (see Fig. 17) is that the individual atoms of Pd are so firmly anchored to the nanoporous walls of the C3N4 that they exhibit minimal tendency to migrate and coalesce to form nanoclusters. This catalyst was shown to be active in a three-phase hydrogenation of alkynes in flow mode, and both its selectivity and activity surpassed those of nanoparticulate Pd. Apart from C3N4, there is now great interest focused on another ‘indented’ (holey) nitrogenous graphene-like carbon of empirical formula C2N. Recent theoretical work done on this catalyst support for single atoms of members of the 3d transition metal series, using an augmented wave version of the density functional theory approach, indicates that Cr and Mn single atoms would catalyse the conversion (by an Eley–Rideal mechanism) of CO and O2 to yield CO2 [42].
(Top) Schematic of a single atom Pd catalyst comprising isolated Pd atoms on a solid support of carbon nitride (C3N4; carbon, grey; nitrogen, purple), which acts as a catalyst for hydrogenation reactions. Strong bonds to the nitrogen atoms firmly anchor the Pd atoms in roughly triangular pores in the stacked, two-dimensional layers of the support. There is approximately one roughly triangular cage per 50 Å2 in each layer, and it is estimated that up to 10 % of them are occupied by a single Pd atom. (Bottom) View parallel to the C3N4 plane showing the density functional theory-optimized position of the Pd atom incorporated in the C3N4 support (After Vilé et al. [40])
In Fig. 18, we show high-resolution images of other few-atom catalytic systems, comprising Au nanoclusters or single atoms supported on TiO2 or activated carbon [43]. Each small spot here is the image of a single metal atom. Christopher and colleagues [44] have also studied isolated metals, in their case, supported on TiO2. They found significant differences in catalytic selectivity between individual atoms of Rh and nanoparticles of the same metal—see Fig. 19. In an elegant assessment of the energetics of single atoms and nanoparticle metal catalysts, namely of Cu on the (111) face of CeO2, Campbell and co-workers [45] were able to arrive at quantitative values of the chemical potential of this metal from the single atom extreme to the bulk state, as shown in Fig. 20. Such work is of great value in ascertaining the long-term thermal stability of single atom catalysts, which is a subject that is now of great relevance.
Aberration-corrected scanning transmission electron microscopy images of nanoclusters of Au on a titania (top) or an activated carbon (bottom) support. Sub-nanometer clusters (white circles), which are approximately 0.5 nm in diameter and contain roughly 10 Au atoms, and individual Au atoms (black circles) are observed (After Thomas et al. [43])
Schematic depiction of different reaction pathways promoted by isolated atoms vs nanoparticles of Rh on TiO2 (After Matsubu et al. [44])
Chemical potential of Cu atoms in Cu nanoparticles on CeO1.95(111) relative to that in bulk Cu(solid), versus the effective diameter of the Cu particle down to the single atom limit (After James et al. [45])
References
Thomas JM (2014) Heterogeneous catalysis and the challenges of powering the planet, securing chemicals for civilised life, and clean efficient utilization of renewable feedstocks. ChemSusChem 7:1801
Thomas JM, Thomas WJ (2015) Principles and practice of heterogeneous catalysis, 2nd edn. Wiley-VCH, Weinheim
Thomas JM (2015) Opportunities for New Catalysts in the Present Confusing Scene in Renewable Energy Green. Int Journal Sustain Energ Conversat Storage 5:55
Thomas JM (2016) Summarizing comments on the discussion and a prospectus for urgent future action. Phil Trans R Soc A 374:20150226
Thomas JM, Harris KDM (2016) Some of tomorrow’s catalysts for processing renewable and non-renewable feedstocks, diminishing anthropogenic carbon dioxide and increasing the production of energy. Energy Environ Sci 9:687
Ampelli C, Perathoner S, Centi G (2015) CO2 utilization: an enabling element to move to a resource- and energy-efficient chemical and fuel production. Phil Trans R Soc A. doi:10.1098/rsta.2014.0177
Dhakshinamoorthy A, Navalon S, Corma A, Garcia H (2015) Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ Sci 5:9217
Krauss C (2016) China’s Hunger for Commodities Wanes, and Pain Spreads Among Producers. International New York Times, Jan 9, 2016
Sastre F, Pago AV, Liu L, Corma A, Garcia H (2014) Complete photocatalytic reduction of CO2 to methane by H2 under solar light irradiation. J Am Chem Soc 136:6798
Hoch LB, Wood TE, O’Brien PG, Liao K, Reyes LM, Mions CA, Ozin GA (2014) The Rational Design of a Single-Component Photocatalyst for Gas-Phase CO2 Reduction Using Both UV and Visible Light. Adv Sci 1:1400013
Langanke J, Wolf A, Hoffman J, Böhn K, Subhani MA, Müller TE, Leitner W, Güntler C (2014) Carbon dioxide (CO2) as sustainable feedstock for polyurethane production. Green Chem 16:1865
Lively RP, Sharma P, McCoal BA, Beaudry-Losique J, Luo D, Thomas JM, Realff M, Chance RR (2015) Anthropogenic CO2 as a feedstock for the production of algal-based biofuels. Biofuels Bioprod Birefin 9:72
Thomas JM (2012) Design and application of single-site heterogeneous catalysts: contributions to green chemistry, clean technology and sustainability. Imperial College Press, London
Erb TJ (2011) Carboxylases in Natural and Synthetic Microbial Pathways. Appl Environ Microbiol 77:8466
Banerjee A, Dick GR, Yoshino T, Kanan M (2016) Carbon dioxide utilization via carbonate-promoted C–H carboxylation. Nature 531:215
Beckman EJ (2016) Sustainable chemistry: putting carbon dioxide to work. Nature 531:180–181
Earhart AJJE, Faaij APC, Patel MK (2012) Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ Sci 5:6407
Freund H-J (2015) Private communication to JMT, 11 Aug 2015
Wurm J, Entwhistle J (2015) Algae-powered architecture. Ingenia 64:30
Thomas JM (2014) Reflections on the topic of solar fuels. Energy Environ Sci 7:19
Jacobson MZ, Delucchi MA et al (2015) 100% clean and renewable wind, water, and sunlight (WWS) all-sector energy roadmaps for the 50 United States. Energy Environ Sci 8:2093
Jacobson MZ, Delucchi MA (2009) A path to sustainable energy by 2030. Sci Am 301:58
Ozin GA (2015) Throwing new light on the reduction of CO2. Adv Mater 27:1957
Ghuman KK, Hoch LB, Szymanshu P, Loh JYY, Kherani NP, Etsayed MA, Ozin GA, Singh CV (2016) Photoexcited Surface Frustrated Lewis Pairs for Heterogeneous Photocatalytic CO2 Reduction. J Am Chem Soc 138:1206
Anpo M, Takeuchi M (2003) The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J Catal 216:505
Anpo M, Thomas JM (2006) Single-site photocatalytic solids for the decomposition of undesirable molecules. Chem Commun 31:3273
Yoo B-K, Su Z, Thomas JM, Zewail AH (2016) On the dynamical nature of the active center in a single-site photocatalyst visualized by 4D ultrafast electron microscopy. PNAS 113:503
Roberts MA, Sankar G, Xu RR, Thomas JM (1996) Synthesis and structure of a layered titanosilicate catalyst with five-coordinate titanium. Nature 381:401
Jia J, O’Brien PG, Ozin GA (2016) Visible and Near-Infrared Photothermal Catalyzed Hydrogenation of Gaseous CO2 over Nanostructured Pd@Nb2O5. Adv Sci. doi:10.1002/advs.201600189
Garcia-Martinez J, Li K, Krishnaiah G (2012) A mesostructured Y zeolite as a superior FCC catalyst – from lab to refinery. Chem Commun 48:11841
Speronello R, Garcia-Martinez J, Hanson A, Hu R (2011) FCC catalysts with mesoporous zeolite yield higher quality products. Refin Oper 2:1
Garcia-Martinez RJ, Li K (ed) (2015) Mesoporous zeolites: preparation, characterization and applications. Wiley-VCH, Weinheim
Zhao C, Lercher JA (2012) Upgrading pyrolysis oil over Ni/HZSM-5 by cascade reactions. Angew Chem Int Ed 51:5935
Zhao C, Brück T, Lercher JH (2013) Catalytic deoxygenation of microalgae oil to green hydrocarbons. Green Chem 15:1720
Boateng AA, Mullen CA, Goldberg NM, Hicks KB, Devine TE, Lima IM, McMurtrey JE (2010) Sustainable production of bioenergy and biochar from the straw of high biomass soybean lines via fast pyrolysis. Env Prog Sus Energy 29:175
Peng B, Yao Y, Zhao C, Lercher HH (2012) Towards quantitative conversion of microalgae oil to diesel-range alkanes with bifunctional catalysts. Angew Chem Int Ed 51:2072
http://www.licella.com.au/. Accessed 10 Aug 2016
Yang X, Wang A, Qian B, Li J, Liu J, Zhang T (2013) Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc Chem Res 46:1740
Flytzani-Stephanopoulos M (2014) Gold atoms stabilized on various supports catalyze the water-gas shift reaction. Acc Chem Res 47:783
Vilé G, Albani D, Nachtegaal M, Chen Z, Wovetsoon D, Antonietti M, Lopez N, Perez-Ramirez J (2015) A stable single-site palladium catalyst for hydrogenations. Angew Chem Int Ed 54:11265
Thomas JM (2015) Catalysis: Tens of thousands of atoms replaced by one. Nature 525:325
Ma DW, Wang Q, Yan X, Zhang X, He C, Zhou D, Tang Y, Lu Z, Yang Z (2016) 3d transition metal embedded C2N monolayers as promising single-atom catalysts: a first-principles study. Carbon 105:463
Thomas JM, Raja R, Gai PL, Grönbeck H, Hernández-Garrido JC (2010) Exceptionally Active Single-Site Nanocluster Multifunctional Catalysts for Cascade Reactions. ChemCatChem 2:402
Matsubu JC, Yang VN, Christopher P (2015) Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J Am Chem Soc 137:3076
James TE, Henmmingson SL, Campbell CT (2015) Energy of Supported Metal Catalysts: from Single Atoms to Large Metal Nanoparticles. ACS Catal 5:5673
Acknowledgments
JMT is grateful to the Kohn Foundation for financial support. RKL is in receipt of a Junior Research Fellowship from Clare College, Cambridge, whose support is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Thomas, S.J.M., Leary, R.K. On choosing the most appropriate catalysts for the conversion of carbon dioxide to fuels and other commodities, and on the environmentally benign processing of renewable and nonrenewable feedstocks. Appl Petrochem Res 6, 167–182 (2016). https://doi.org/10.1007/s13203-016-0167-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-016-0167-9