Abstract
The present study aimed to evaluate the levels of acrylamide in drinking water by solid phase extraction (SPE). After SPE, water extracts were analyzed using gas chromatography–mass spectrometry (GC–MS) system. Thirty drinking water samples were analyzed in the study. Acrylamide was detected in 12 samples. The levels of acrylamide in drinking water samples were less than 85 ng l−1. The limit of detection and limit of quantification were 4 and 13.20 ng l−1, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acrylamide (C3H5NO, prop-2-enamide, Mw: 71.08 g mol−1) is a small chemical compound used for over 50 years; now that is used mainly for the monomer to synthesize polyacrylamides and its copolymers (EHC 49 1985). Acrylamide is used as flocculants to purifying drinking water and wastewater (Friedman 2003).
It is used in many several industrial sectors such as paper, plastics, textile, dyes and cosmetics (Alpmann and Morlock 2008). The high water solubility of acrylamide causes acrylamide to be present in drinking water. Acrylamide is one of the chemical structures of the carcinogen group (IARC 1994, 1997). The World Health Organization (WHO) has stated that the guideline set value associated with the maximum concentration of acrylamide in drinking water is 0.5 µg l−1 (WHO 1991, 1993, 2011). Also, restrictive quality requirement limits of 0.1 µg l−1 for water intended for human consumption have been regulated in European Union and take part in the EU 98/83/EC Drinking Water Directive (Council Directive 98/83/EC 1998). Animal studies have shown that acrylamide has a negative effect on health (Johnson et al. 1986; Neumann 1991; Alison et al. 1994; Friedman et al. 1995; Ben-Jonathan et al. 2008; Beland et al. 2013).
To date, several analytic methods for the analysis of acrylamide in water have typically involved chromatographic (liquid chromatography, gas chromatography) separation followed by spectrophotometric detector [ultraviolet (UV) detector, diode array detector (DAD), fluorescence detector], electron capture detector (ECD) or mass spectrometry (Cavalli et al. 2004; Güven and Gezgin 2005; Alpmann and Morlock 2008; Kepekci et al. 2012; Yamini et al. 2012; Lim and Shin 2013; Backe et al. 2014; Sobhi et al. 2017). Most analysis of acrylamide achieved in experiments to date has been based on derivatization or without derivatization (Cavalli et al. 2004; Güven and Gezgin 2005; Alpmann and Morlock 2008). The extraction of acrylamide from water samples is usually achieved via hollow fiber liquid phase microextraction (Sobhi et al. 2017), liquid–liquid extraction (Güven and Gezgin 2005; Backe et al. 2014) and solid phase extraction (Alpmann and Morlock 2008).
The aim of the study is to make acrylamide analysis in 30 drinking water samples using SPE method by GC–MS without the derivation step.
Materials and methods
Chemicals, reagents and material
Methanol, acrylamide and acetone were obtained from Merck and Sigma-Aldrich. Restek™ CarboPrep Cartridges were used for solid phase extraction.
Drinking water samples
Drinking water samples were taken from different provinces in Burdur and Isparta. Bottled water samples of different brands were purchased from local markets in Burdur. In this study, a total of 30 water samples were analyzed.
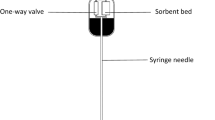
SPE cleanup procedure
Restek™ CarboPrep cartridges (6 ml, 500 mg) were placed in the manifold system and activated with 2 ml acetone and finally rinsing 2 ml 0.1% formic acid. The water sample (about 250 ml) was loaded on the column and the sorbents were dried. Acrylamide was eluted from the cartridges using 2 ml acetone (Acrylamide analysis by gas Chromatography 2004).
Chromatography and apparatus
An Agilent 7890A GC equipped with a 5975 MS detector, a 7693B automatic sampler and a MSDCHEM (Agilent, USA) data system was used for the determination of acrylamide in the analyzed water samples. GC temperature program and operating conditions are given in Table 1 (Agilent Technologies Inc 2014).
Results and discussion
Method parameters
Stock standard solution of acrylamide was prepared in acetone. Six different concentrations are used for calibration curves. Linear regression analysis was carried out by plotting peak area (y) versus acrylamide concentration (x). A signal-to-noise (S/N) ratio of 3:1 is generally considered suitable ratio for the LOD. A typical S/N ratio is 10:1 for a LOQ. The extraction recovery was determined by spiking samples with acrylamide in three replicates; they were extracted as previously described. The LOD and LOQ were calculated to be 4.00 and 13.20 ng l−1, respectively.
This value was adequate for the monitoring of acrylamide at the limit value of 0.5 µg l−1 as demanded by WHO and EPA and 0.1 µg l−1 in drinking water as stipulated by EU. In different studies, LOD values have been reported between 1–200 ng l−1 (Cavalli et al. 2004; Lim and Shin 2013; Kepekci et al. 2012; Backe et al. 2014; Yamini et al. 2012; Sobhi et al. 2017; Alpmann and Morlock 2008). The LOD and recovery values obtained in this study are similar to those in the literature. Precision is a measure of the degree of reproducibility/reproducibility of the analytical method tested under the working conditions. Repeatability was measured using three different concentrations of acrylamide prepared and analyzed. Intraday and interday instrument variations were examined to determine the sensitivity of the proposed analytical methods (Table 2). RSD values obtained from intraday and interday studies are lower than 5%.
The accuracy of the analytical method being tested is expressed as the proximity of the measured value to the actual value for the sample. In our study, acrylamide was added to the water sample (Table 3). For the accuracy studies, the sample preparation was carried out exactly. Accuracy is generally expressed as the standard deviation or percent relative error and the average mean recovery % of the analytical method (Table 4).
When Restek™ CarboPrep cartridge was used, average percent recovery values were higher than 86.83 (± 1.85)%. Relative standard deviation (RSD) values obtained from recovery studies are also lower than 5%. In different studies, recovery studies for acrylamide were made from different water samples. The recovery value 89–113% for acrylamide was reported (Cavalli et al. 2004; Lim and Shin 2013; Kepekci et al. 2012; Backe et al. 2014; Yamini et al. 2012; Sobhi et al. 2017; Alpmann and Morlock 2008).
Analytical results
The content of acrylamide in various drinking water samples was investigated in Table 5.
The level of acrylamide in drinking water samples ranged from 14.53 to 85.00 ng l−1. The concentrations of acrylamide detected at the drinking water studied currently were well below the 0.1 µg l−1 and 0.5 µg l−1 limits required by the EU and EPA. Igisu et al. (1975) reported that mean acrylamide concentration was 400 mg l−1. Brown and Rhead (1979) have reported the acrylamide level in sea water was 3.4 µg l−1.
Conclusions
This study was done without derivatization. The level of acrylamide in drinking water samples analyzed in this study. The LOD was calculated to be 4.00. This value was adequate for the monitoring of acrylamide at the limit value of 0.5 µg l−1 as demanded by WHO and EPA and µg l−1 in drinking water as stipulated by EU. Acrylamide was detected in 12 samples. The amount of acrylamide in the samples outside the table was below the detection limit. Thirty drinking water samples were analyzed in the study. Acrylamide was detected in 12 samples.
References
Acrylamide analysis by gas Chromatography (2004) Perkin Elmer Field Application Report 1-3. https://www.perkinelmer.com/CMSResources/Images/44-74337FAR_GCAcrylamideAnalysis.pdf
Agilent Technologies Inc (2014) 5991-5297EN procedure. https://www.agilent.com/cs/library/applications/5991-5297EN_v2.pdf. Accessed Oct 2014
Alison RH, Capen CC, Prentice DE (1994) Neoplastic lesions of questionable significance to humans. Toxicol Pathol 22(2):179–186
Alpmann A, Morlock G (2008) Rapid and sensitive determination of acrylamide in drinking water by planar chromatography and fluorescence detection after derivatization with dansulfonic acid. J Sep Sci 31:71–77. https://doi.org/10.1002/jssc.200700391
Backe WJ, Yingling V, Johnson T (2014) The determination of acrylamide in environmental and drinking waters by large-volume injection–hydrophilic-interaction liquid chromatography and tandem mass spectrometry. J Chromatogr A 1334:72–78. https://doi.org/10.1016/j.chroma.2014.02.005
Beland FA, Mellick PW, Olson GR, Mendoza MC, Marques MM, Doerge DR (2013) Carcinogenicity of acrylamide in B6C3F (1) mice and F344/N rats from a 2-year drinking water exposure. Food Chem Toxicol 51:149–159. https://doi.org/10.1016/j.fct.2012.09.017
Ben-Jonathan N, LaPensee CR, LaPensee EW (2008) What can we learn from rodents about prolactin in humans. Endocr Rev 29:1–41. https://doi.org/10.1210/er.2007-0017
Brown L, Rhead M (1979) Liquid chromatographic determination of acrylamide monomer in natural and polluted aqueous environments. Analyst 104:775–778
Cavalli S, Polesello S, Saccani G (2004) Determination of acrylamide in drinking water by large-volume direct injection and ion-exclusion chromatography–mass spectrometry. J Chromatogr A 1039(1–2):155–159
Council Directive 98/83/EC of 3 November 1998 on the Quality of water intended for human consumption. OJ L 330, 5.12.1998, 32–5
Friedman M (2003) Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem 51:4504–4526. https://doi.org/10.1021/jf030204+
Friedman MA, Dulak LH, Stedham MA (1995) A lifetime oncogenicity study in rats with acrylamide. Fundam Appl Toxicol 27(1):95–105
Güven KC, Gezgin T (2005) Determination of acetonitrile and detection of acrylmaide in seawater by GC/MS. Acta Pharmaceutica Turcica 47:15–20
Igisu H, Goto I, Kawamura Y, Kato M, Izumi K, Kuroiura Y (1975) Acrylamide encephaloneuropathy due to well water pollution. J Neurol Neurosurg Psychiatry 38:581–584
International Agency for Research on Cancer (1994) Some industrial chemicals. IARC Monogr Eval Carcinog Risks Hum 60:389–433
International Agency for Research on Cancer (1997) IARC Monographs on the evaluation of carcinogenic risks to humans, vol. 60, Lyon 1994, updated 1997
Johnson KA, Gorzinski SJ, Bodner KM, Campell RA, Wolf CH, Friedman MA, Mast RW (1986) Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol Appl Pharmacol 85(2):154–168
Kepekci TS, Önal C, Önal A (2012) A review of current methods for the determination of acrylamide in food products. Food Anal Methods 5(1):29–39. https://doi.org/10.1007/s12161-011-9277-2
Lim H, Shin H (2013) Ultra-trace level determinations of acrylamide in surface and drinking water by GC–MS after derivatization with xanthydrol. J Sep Sci 36(18):3059–3066
Neumann F (1991) Early indicators for carcinogenesis in sex-hormone-sensitive organs. Mutat Res 248(2):341–356
Sobhi HR, Ghambarian M, Behbahani M, Esrafili A (2017) Application of modified hollow fiber liquid phase microextraction in conjunction with chromatography–electron capture detection for quantification of acrylamide in waste water samples at ultra-trace levels. J Chromatogr A 1487:30–35. https://doi.org/10.1016/j.chroma.2017.01.051
Word Health Organization (WHO) (1991) Acrylamide health and safety guide. Health and safety guide no: 45, Geneva
Word Health Organization (WHO) (1993) Guidelines for drinking water quality recommendations, vol 1, 2nd edn. WHO, Geneva
World Health Organızatıon (WHO) (2011) Acrylamide in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality. http://www.who.int/water_sanitation_health/dwq/chemicals/acrylamide.pdf
Yamini Y, Ghambarian M, Esrafili A, Yazdanfar N, Moradi M (2012) Rapid determination of ultra-trace amounts of acrylamide contaminants in water samples using dispersive liquid–liquid microextraction coupled to gas chromatography-electron capture detector. Int J Environ Anal Chem 92:1493–1505. https://doi.org/10.1080/03067319.2010.548098
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Seçilmiş Canbay, H., Doğantürk, M. Analysis of acrylamide in drinking water by SPE and GC–MS. Appl Water Sci 9, 42 (2019). https://doi.org/10.1007/s13201-019-0918-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-0918-8