Abstract
In the present study, the agricultural residues viz., Syzygium cumini and Populus deltoides leaves powder have been used for the biosorption of Cu(II), Ni(II), and Cr(VI) from aqueous solutions. FTIR and SEM analysis of the biosorbents were performed to explore the type of functional groups available for metal binding and to study the surface morphology. Various physico-chemical parameters such as pH, adsorbent dosage, initial metal ion concentration, and equilibrium contact time were studied. Thermodynamic studies were carried out and the results demonstrated the spontaneous and endothermic nature of the biosorption process. The equilibrium data were tested using four isotherm models—Langmuir, Freundlich, Temkin and Dubinin–Radushkevich and the maximum biosorption capacities were evaluated. The Pseudo-first-order, pseudo-second-order, Elovich and intraparticle diffusion models were applied to study the reaction kinetics with pseudo-second order model giving the best fit (R2 = 0.99) to the experimental data.
Similar content being viewed by others
Introduction
Heavy metal contamination of stream and river water ecosystem is a worldwide problem. High concentration of heavy metals in the environment can be detrimental to a variety of living organisms. Excessive intake of these metals by humans can cause accumulative poisoning, cancer, nervous system damage and ultimately death. The main industries responsible for this are electroplating, electronics, batteries, paint industry, paper industry and metal fabrication. Though some of the heavy metals are essential in trace amounts, at higher concentrations they are highly lethal as they cause some incurable diseases such as higher concentrations of chromium lead to liver damage, pulmonary congestion, oedema and skin irritation resulting in ulcer formation (Raji and Anirudhan 1998). The effects of Ni exposure vary from skin irritation to damage to the lungs, mucous membranes and nervous system (Oliver 1997). Excessive human intake of Cu leads to severe mucosal irritation and corrosion, widespread capillary damage, hepatic and renal damage and central nervous system irritation followed by depression (Ajmal et al. 1998). To minimize these potential health problems, the United States Environmental Protection Agency has set the limits for these heavy metals in drinking water (USEPA 2006). It is 1.3 mg/L for copper, 0.1 mg/L for nickel and 0.05 mg/L for chromium. Thus, it is an issue of utmost concern to give proper treatment to industrial effluent so that it becomes pollution free by the time it is disposed off into the aqueous systems.
A number of methods were opted for the removal of metal ions from aqueous solutions (Alothman and Apblett 2009, 2010). These include ion exchange (Petruzzelli et al. 1999), solvent extraction (Gupta et al. 2003) reverse osmosis, electrodialysis (Hasar 2003), precipitation (Remoudaki et al. 2003), flocculation (Zhao et al. 2005), sorption (Sharma et al. 1991), activated carbon adsorption and membrane separation processes (Yan and Viraraghavan 2001). However, these techniques have one major drawback that they involve high capital and operational cost. Among these, biosorption has proved to be a potential alternative to these techniques due to its high efficiency, ease of operation, simplicity of design, and comparable low cost (Chowdhury and Saha 2010; Saha et al. 2010). Though commercial activated charcoal has proved its worth as an efficient adsorbent, its higher cost limits its wider application (Babel and Kurniawan 2003). Thus, different forms of inexpensive, easily available and effective biosorbents such as waste acorn of cashew nut shell (Kumar et al. 2012), sugar cane dust (Mondal et al. 2011), lotus stalks (Huang et al. 2010), pine cone activated carbon (Momčilović et al. 2011), Tamarindus indica (Chowdhury and Saha 2011), gum karaya (Sterculia urens) (Vinod et al. 2011), fluted pumpkin seed shell (Okoye et al. 2010), Melocanna baccifera (Lalhruaitluanga et al. 2011) have been widely used as potential adsorbents for heavy metals.
In this study, series of batch experiments were carried out to assess the potentiality of Syzygium cumini and Populus deltoides leaves powder for the removal of Cr(VI), Ni(II) and Cu(II) from aqueous solutions. The effects of various parameters viz., pH, adsorbent dose, initial metal ion concentration, temperature and contact time on the biosorption capacity of both the adsorbents were investigated. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherm models were applied to the experimental data and metal uptake capacity (qe) was calculated. Thermodynamic and kinetics were also investigated and reported.
Materials and methods
Preparation of biosorbents
The fallen leaves of S. cumini and P. deltoides were collected from Ambala (India). The collected leaves were washed separately with running tap water several times to remove the dirt and other adhering particulate matter. The washed leaves were dried in the sunlight for 7 days till they dried completely. The dried leaves were then ground and screened through a mesh size of 0.6 mm (Singh and Ali 2012). The resulted uniform powder was treated with 1.0 M HCl solution and stirred at 100 °C for 24 h to remove the coloring pigments. The powder obtained was then washed repeatedly with deionised water to bring the pH to neutral and finally dried in a hot air oven. The dried S. cumini leaves powder (SCLP) and P. deltoides leaves powder (PDLP) thus obtained were stored in separate airtight plastic containers for further use.
Preparation of stock solutions
All the chemicals used were of AR grade. Stock solutions (1,000 mg L−1) of Cr(VI), Ni(II) and Cu(II) ions were prepared by dissolving potassium dichromate (K2Cr2O7), nickel nitrate hexahydrate and copper sulphate (CuSO4·5H2O), respectively, in deionised water. The stock solutions were diluted further with deionised water to prepare the solutions of the desired concentrations and the pH of the solutions was adjusted by adding 0.1 N HCl or 0.1 N NaOH solutions.
Instruments and software
The quantification of the metal ions in the solutions has been performed on AAS (AA630, Shimadzu, Japan). FTIR spectra of the adsorbent were recorded on Thermo, Nicolet 10 FTIR spectrophotometer and field emission scanning electron microscopy (FESEM) was performed on JEOL JSM 6510LV to collect the SEM images. The pH of the solution has been measured by Cyber scan, Eutch pH meter and the orbital shaker incubator by Metrex scientific instruments has been used for shaking the samples during batch operations at desired temperature and rpm. Software Sigma plot 11 has been used for the data analysis and fitting the experimental data.
Batch experiments
The batch experiments were carried out in 250-mL glass stoppered, Erlenmeyer flasks with 100 mL of working volume and 50 mg L−1 metal ion concentration. 1.0 g of the biosorbent was added to the solution. The flasks were agitated at constant speed of 225 rpm for 12 h in an incubator shaker at 30 °C. The influence of pH (1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0), adsorbent dose (0.5, 1.0, 1.5, 2.0 g L−1), initial metal ion concentration (50, 100, 150, 200 mg L−1) and temperature (283, 288, 293,298, 303, 308 K) was evaluated during the present study. Samples were collected from the flasks at predetermined time intervals for analyzing the residual Cr(VI), Ni(II) and Cu(II) concentration in the solution using atomic absorption spectrophotometer.
The amount of metal adsorbed at equilibrium qe (mg g−1) was calculated according to a mass balance on the metal ion concentration using Eq. (1):
where Ci and Ce are the initial and equilibrium metal ion concentration, respectively, in solution (mg L−1) of Cr(VI), Ni(II) and Cu(II), V is the volume of the solution (L), and m is the mass of the adsorbent used (g).
The percent removal (%) of Cr(VI), Ni(II) and Cu(II) were calculated using Eq. (2):
To ensure the accuracy, reliability, and reproducibility of the collected data, all biosorption experiments were performed in triplicate, and the mean values were used in data analysis.
In a biosorption study, it is necessary to fit the equilibrium biosorption data using different biosorption isotherm models and kinetic equations in order to analyze and design a biosorption process. Therefore, different theoretical models (Table 1) were applied to the experimental data in order to find which model adequately predicts isotherm and kinetic study.
Point-of-zero charge
The charge on the biomass surface is a function of pH. The pH with which the charge of the solid surface is zero is referred to as the point-of-zero charge (pHpzc) (Rivera-Utrilla et al. 2001). The determination of the pHpzc of the samples was carried out using a procedure similar as described by Rivera et al.: 50 ml of 0.01 M NaCl solutions were taken in 100-mL screw-cap conical flasks. The pH of each solution in each flask was adjusted to values of 2, 4, 6, 8, 10 and 12 by adding 0.1 M HCl or NaOH solutions. Then, 0.15 g of both SCLP and PDLP were added separately and the final pH measured after 48 h under agitation at room temperature. The pHpzc is the point where the curve pH final versus pH initial crosses the line equal to pH final (Rivera-Utrilla et al. 2001).
Regeneration of the exhausted biosorbent
Desorption experiments were carried out using 0.1 N HCl and 0.1 N NaOH solutions as the stripping agents. Metal-loaded biosorbents obtained from our sorption experiments were transferred to the Erlenmeyer flasks and shaken for 24 h. The filtrate was analyzed for desorbed Cr(VI), Ni(II) and Cu(II) ions. Concentration of the metal ions in the solution corresponded to the amount of metal desorbed from the adsorbent and enabled to determine the percentage fraction of desorption. Consecutive biosorption–desorption cycles were repeated thrice by using the same sorbents.
Characterization of electroplating industry waste water
Electroplating industry waste water was collected from the electroplating unit situated in Karnal, Haryana, India, and analyzed. The details are given in Table 2.
Packed bed column experiments
Among SCLP and PDLP, PDLP showed good adsorption capacity for all the three metal ions in batch mode and hence it was used in column studies for treating electroplating industry waste water. Continuous flow experiments were conducted in three glass columns (internal diameter = 1.3 cm, length = 50.0 cm) filled with PDLP up to the 20.0, 25.0 and 30.0 cm bed heights. The packing of PDLP was done with proper care to avoid any void spaces, channels and cracks in the bed. The PDLP-packed columns were held vertically with the help of stand and clamp, washed thrice with deionised water. Then electroplating waste water was passed through all the columns at flow rate of 2.0 mL min−1 using peristaltic pump. Effluents were collected in conical flask in 20.0-mL fractions and analyzed for the residual metal ion concentration using atomic absorption spectrophotometer.
Results and Discussion
Characterization of biosorbent
FTIR analysis
The biosorption of metal ions onto plant materials is attributed to the active groups and bonds present on them. FTIR analysis was employed to investigate the major functional groups on the biosorbent surface which are responsible for biosorption of metal ions. FTIR spectra obtained for SCLP and PDLP samples before and after biosorption process are shown in Figs. 1 and 2, respectively. Peaks appearing in the FTIR spectrum of native SCLP and PDLP were assigned to various groups and bonds in accordance with their respective wave numbers (cm−1). The broad peak between 3,440 and 3,450 cm−1 was assigned to the presence of free or hydrogen bonded O–H groups (from carboxylic acids or alcohols) on the surface of the adsorbents. The two peaks appearing between 2,850 and 2,920 cm−1 represent the asymmetrical and symmetrical stretching vibration of methylene (–CH2) groups due to C–H bonds of aliphatic acids. The peaks around 1,500–1,652 cm−1 suggest that aromatic ring bands and double bond (C=C) vibrations overlap with C=O stretching vibration bands and OH bending vibration bands. The peaks near 1,650 cm−1 arises from C=O stretching in amide groups. The band between 1,020 and 1,040 cm−1 can be assigned to C–O stretching vibration of alcohols and carboxylic groups. It is well indicated from FTIR spectrum that carboxyl and hydroxyl groups are present in abundance and are responsible for binding with metal ions (Ashkenazy et al. 1997).
After biosorption of the metal ions, the band at 3,450 cm−1 corresponding to O–H groups shifts to the lower frequency 3,420 cm−1. Thus, it can reasonably concluded that carboxyl and hydroxyl groups may be the main groups responsible for binding metal ions to both the adsorbent surfaces.
Scanning electron microscopy
SEM is one of the most useful techniques to study the morphological features and surface characteristics of the biosorbent. The SEM images of SCLP and PDLP before and after biosorption are shown in Figs. 3 and 4, respectively. The surface of the biosorbents was rough, uneven and had rod clusters before adsorption. The rough surface can help to increase the surface area available for biosorption of metal ions. But it becomes smooth after biosorption due to the filling of the binding sites with Cr(VI), Ni(II) and Cu(II) metal ions. The surface morphological change can be linked to precipitation/complexation of metal ions onto the biosorbent surface.
Batch studies
Effect of pH
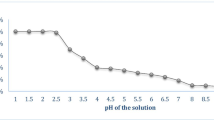
The solution pH is an important parameter influencing heavy metal biosorption from aqueous solutions. It affects both, the surface charge of the adsorbent and the degree of ionization of the heavy metal in solution (Aksu 2005). The effect of pH on the biosorption of Cr(VI), Ni(II) and Cu(II) on SCLP and PDLP was studied by varying pH range from 1.0 to 7.0 with adsorbent dose 1.5 g, 50 mg L−1 initial metal ion concentration and temperature 30 °C at 225 rpm. Cr(VI) was strongly adsorbed at pH 2.0 (Fig. 5), while Ni(II) and Cu(II) were adsorbed at pH 6.0 and 4.0, respectively. This can be explained with the help of pHPZC of the adsorbent. pHPZC is the pH value, at which the solid surface of the adsorbent has the net zero charge. The points of zero charges (pHPZC) of both SCLP and PDLP were experimentally found to be at pH 2.0, 2.12, respectively (Fig. 6). Further, at pH > pHPZC the adsorbent became negatively charged and the metal species were positively charged. Under such circumstances, the electrostatic attraction between the positively charged metal ions and the negatively charged adsorbent surface increases resulting in enhanced adsorption of the metal ions from the solution. On the other hand, at pH < pHPZC the surface of the adsorbent became positively charged resulting in a decrease in the metal ions adsorption apparently due to the higher concentration of H+ ions in the solution that were challenging the positively charged metal for the active sites. Further biosorption experiments were carried out at pH 2.0, 6.0 and 4.0 for Cr(VI), Ni(II) and Cu(II) metal ions, respectively.
Effect of adsorbent dosage
The biosorption profile of Cr(VI), Ni(II) and Cu(II) versus different SCLP and PDLP adsorbent dosage in the range of 0.5–2.0 g was also determined (Fig. 7). It was observed that the percentage removal of all three metal ions increased with increase of adsorbent dose, reaching maximum at around 1.5 g, while the loading capacity (amount of metal ion loaded per unit weight of the adsorbent) gradually decreased. The positive correlation between adsorbent dose and Cr(VI), Ni(II) and Cu(II) removal can be related to increase in adsorbent surface area and availability of more biosorption sites (Babel and Kurniawan 2004). Further increase in adsorbent dose did not significantly change the biosorption yield. This is due to the binding of almost all metal ions to the adsorbent surface and establishment of equilibrium between the metal ions on the adsorbent and in the solution (Garg et al. 2003; Pons and Fuste 1993).
Effect of initial metal ion concentration
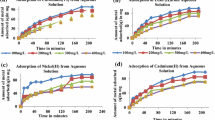
In batch biosorption processes, the rate of biosorption is a function of the initial concentration of metal ions, which makes it an important factor for effective biosorption. The percentage metal removal at different initial metal ion concentration (50–200 mg L−1) of Cr(VI), Ni(II) and Cu(II) using SCLP and PDLP is shown in Fig. 8. The percentage removal of metal ion decreases with increase in initial metal concentration and shows little decrease at higher concentrations. This can be explained by the fact that the adsorbent has a limited number of active sites that become saturated at a certain concentration (Aksu and Donmez 2003). Although the percentage removal of metal ion decreases, the equilibrium biosorption capacity of the adsorbent increases with increasing metal ions concentration. The initial metal ion concentration provides an important driving force to overcome all the mass transfer resistance between the solution and solid phases, hence a higher initial concentration of metal ion may increase the biosorption capacity.
Biosorption thermodynamics
The biosorption of metal ions on SCLP and PDLP was investigated as a function of temperature. The batch experiments were performed by varying the temperature from 283 to 308 K with fixed initial Cr(VI), Ni(II) and Cu(II) concentration of 50 mg L−1 at pH 2.0, 6.0 and 4.0, respectively, and adsorbent dose of adsorbents 1.5 g L−1 of both the adsorbents. The equilibrium metal ion biosorption capacity of the both SCLP and PDLP was better at higher temperature and this could be attributed to the increase in molecular diffusion or to the availability of more active sites on the surface of the SCLP and PDLP by expansion of the pores at the same temperature (Singh and Ali 2012).
Thermodynamic parameters such as free energy (ΔG°), enthalpy (ΔH°), and entropy (ΔS°) change of biosorption can be evaluated from the following equations:
where R is the gas constant (8.314 J mol−1 K−1), T is the temperature (K) and K d is the equilibrium constant. The value of K d was calculated using Eq. (4):
where qe and Ce are the equilibrium concentrations of metal ions on the adsorbent and in the solution, respectively.
Also we know that
Equations (3) and (5) can be combined together as:
A plot between lnK d versus 1/T is shown in (Fig. 9) for both the adsorbents. The values of ΔH° and ΔS° can be calculated from slope and intercept, respectively, and the values of ΔG° were calculated from Eq. (5).
The negative value of ΔG° (Tables 3, 4) at all temperatures confirms the spontaneous nature and feasibility of the biosorption process for Cr(VI), Ni(II) and Cu(II) onto SCLP and PDLP. The positive value of ΔH° confirms that process is endothermic in nature. The positive value of entropy (ΔS°) shows increased randomness at the solid–solution interface during the biosorption process (Kelleher et al. 2002). The adsorbed water molecules, which are displaced by the adsorbate species, gain more translational entropy that is lost by the adsorbate molecules, thus allowing prevalence of randomness in the system (Kelleher et al. 2002).
Adsorption isotherms
An adsorption isotherm is a good tool for understanding the nature of the surface of the biosorbents. However, a correct selection of biosorption equation for different concentration ranges reveals a true picture of the surface.
The biosorption data have been subjected to different biosorption isotherms, namely, Langmuir, Freundlich, Temkin and Dubinin–Kaganer–Radushkevich (DKR) (Table 4).
The Langmuir isotherm constant KL and qmax were calculated from the slope and intercept of the plot Ce/qe versus Ce. The maximum Cr(VI), Ni(II) and Cu(II) sorption onto SCLP was 16.07, 15.60, 15.87 mg g−1 and on PDLP was 18.31, 16.45 and 17.76 mg g−1, respectively, with high value of correlation coefficient (R2). This indicates a good agreement between the parameters and confirms the monolayer biosorption of metal ions on the surface of both the adsorbents.
A further analysis of the Langmuir equation can be made on the basis of dimensionless equilibrium parameter, R L (Hall et al. 1966) also known as separation factor, given by
The value of R L lies between 0 and 1 indicating that the biosorption is favorable for both the adsorbents.
The Freundlich constants KF and n were calculated from the slope and intercept of the straight line plot lnqe versus lnCe. As it can be seen in Table 5, the values of n lies between 1 and 10 (i.e. 1/n < 1), representing a favorable sorption.
Temkin isotherm model was applied and the constants A, b were calculated from the slope and intercept of the plot qe versus lnCe (Table 5). The value of R2, A and b showed that model favors the biosorption of metal ions on SCLP and PDLP.
In order to estimate the characteristic porosity of the biomass and apparent energy of biosorption, the Dubinin–Radushkevich model was used (Dubinin 1960). β is related to the free energy of sorption per mole of sorbate as it migrates to the surface of the biomass from infinite distance in the solution. The porosity parameter values β for the biomass towards the metal was less than unity indicating that sorption of Cr(VI), Ni(II) and Cu(II) onto SCLP and PDLP was significant (Table 5). Values of q m were also in accordance with the experimental data. R2 values obtained from data also supported the fitness of model to sorbents.
In comparing the linear correlation coefficients of all the four isotherms (Table 5), it could be concluded that the biosorption of Cr(VI), Ni(II) and Cu(II) onto SCLP and PDLP was best fitted to Langmuir isotherm under the concentration range studied confirming the monolayer biosorption.
Adsorption kinetics
Pseudo-first-order, pseudo-second-order, Elovich equation and intraparticle diffusion models were applied to study the reaction pathways and potential rate limiting steps of the biosorption of Cr(VI), Ni(II) and Cu(II) onto SCLP and PDLP.
Constants of pseudo-first-order, pseudo-second-order, were determined from the slope and intercept of the linear plot between log (qe − q t ) versus t and t/q t versus t, respectively (Table 6). Correlation coefficient for pseudo-first order was found to be appreciably high, but the calculated qe is not equal to experimental qe, suggesting the insufficiency of pseudo-first-order model to fit within the kinetic data. Pseudo-second-order equation showed excellent linearity with the experimental data with high correlation coefficient (R2 > 0.99) and the theoretical qe value is closer to the experimental qe value. So it was inferred that biosorption of Cr(VI), Ni(II) and Cu(II) onto SCLP and PDLP followed pseudo-second-order kinetics. This suggests that the rate limiting step of this sorption system may be chemisorption involving valency forces through sharing or exchange of electrons between adsorbent and adsorbate. A similar phenomenon was reported in the literature. (Jayaram and Prasad 2009; Lawal et al. 2010). Further, Elovich equation also supports chemisorption processes with high value of α and R2 obtained from the plot of q t versus lnt yielding a straight line.
In many biosorption processes, the adsorbate species are most probably transported from the bulk of the solution into the solid phase through intraparticle diffusion which is often the rate limiting step in many biosorption processes. So the intraparticle diffusion is another kinetic model which was used to study the rate of Cr(VI), Ni(II) and Cu(II) biosorption on SCLP and PDLP. According to this model, if the plot of q t versus t0.5 gives a straight line, then the biosorption process is controlled by intraparticle diffusion. k p can be calculated from the slope of the plot of q t versus t0.5. Values of correlation coefficient are closer to unity which indicates the applicability of this model. The values of intercept give an idea about boundary layer thickness, i.e., the larger intercept, the greater is the boundary layer effect. The applicability of intraparticle diffusion model indicates that it is the rate limiting step (Kannan and Sundaram 2001).
In nutshell, it can be concluded that along with chemisorption, intraparticle diffusion are the rate limiting steps in the biosorption of Cr(VI), Ni(II) and Cu(II) biosorption on SCLP and PDLP.
Regeneration of the exhausted biosorbent
In this study 0.1 N HCl and 0.1 N NaOH were selected to desorb Cr(VI), Ni(II) and Cu(II) from exhausted SCLP and PDLP. Low desorption was obtained with NaOH as compared to HCl indicating that in acidic conditions, heavy metal cations are displaced by protons from the binding sites. Further the regeneration efficiency decreased from 92.63 % (in first cycle) to 45.23 % (in third cycle) in case of Cr(VI) for SCLP and from 76.11 to 35.85 % for PDLP. Similar was the case for Ni(II) and Cu(II) metal ions. The complete desorption of the above said metal ions could not be obtained even with 0.5 N HCl, which might be due to the metal ions being trapped in the intrapores and therefore difficult to release (Al-Asheh and Duvnjak 1997).
Packed bed column experiments
Electroplating waste water containing Cr(VI), Ni(II) and Cu(II) with other anions were passed through three different columns with bed height 20.0, 25.0 and 30.0 cm with 2.0 mL min−1 flow rate. Figure 10 represents the breakthrough curves at different bed heights and it was observed that all the three metals ions uptake capacity increases with increase in bed height. The column with bed height 30.0 cm showed the maximum removal of all the metals ions as compared to other two columns with bed height of 20.0, 25.0 cm. It may be attributed to increased surface area of the adsorbent, which provided more binding sites for the sorption. Along with this the breakthrough time also increases with increase in bed height resulting prolonged saturation for higher bed height columns.
Conclusion
The present investigation showed that both SCLP and PDLP were novel and promising low-cost biosorbents to be used in the removal of Cr(VI), Ni(II) and Cu(II) from aqueous medium over a wide range of concentrations. The following conclusions are made based on the results of the present study:
-
The biosorbent was characterized by Fourier transform infrared (FTIR) spectroscopy and scanning electron microscopy (SEM) techniques.
-
The percentage removal of Cr(VI), Ni(II) and Cu(II) was found to decrease with increase in initial concentration while acidic solution pH 2.0 was more favorable for the adsorption of Cr(VI), 6.0 for Ni(II) and 4.0 Cu(II) on SCLP and PDLP.
-
When the biosorbent dosage was increased, the equilibrium adsorption capacity (mg g−1) of adsorbents gradually decreased, whereas the percent removal efficiency increased.
-
The results showed good correlation coefficients and agreement between experimental and calculated values of qe and pseudo-second-order kinetic mode gave the best fit (R2 = 0.99). The results of the intraparticle diffusion model suggest that intraparticle diffusion along with chemisorption was the rate controlling step.
-
Equilibrium data were fitted to linear models of Langmuir, Freundlich, Temkin and Dubinin–Radushkevich, and the equilibrium data were best described by the Langmuir isotherm model with maximum biosorption capacity of 18.31, 16.45 and 17.76 mg g−1 for Cr(VI), Ni(II) and Cu(II), respectively, on PDLP.
-
The electroplating waste water containing Cr(VI), Ni(II) and Cu(II) was treated using PDLP with different bed heights and it was found that the column with maximum bed height (30.0 cm) was able to remove all three metal ions.
Based on all the above results, it can be concluded that both SCLP and PDLP are effective and alternative biosorbents for the removal of Cr(VI), Ni(II) and Cu(II) from aqueous medium in terms of high biosorption capacity, abundantly available in nature at low cost.
References
Ajmal M, Khan AH, Ahmad S, Ahmad A (1998) Role of sawdust in the removal of Cu(II) from industrial waste. Water Res 32:3085–3091
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. J Process Biochem 40:997–1026
Aksu Z, Donmez G (2003) A comparative study on the adsorption characteristics of some yeasts for remazol blue reactive dye. Chemosphere 50:1075–1083
Al-Asheh S, Duvnjak Z (1997) Sorption of cadmium and other heavy metals by pine bark. J Hazard Mater 56:35–51
Alothman ZA, Apblett AW (2009) Preparation of mesoporous silica with grafted chelating agents for uptake of metal ions. Chem Eng J 155(3):916–924
Alothman ZA, Apblett AW (2010) Metal ion adsorption using polyamine-functionalized mesoporous materials prepared from bromopropyl-functionalized mesoporous silica. J Hazard Mater 182(1–3):581–590
Ashkenazy R, Gottlieb L, Yannai S (1997) Characterization of acetone-washed yeast biomass functional groups involved in lead biosorption. Biotechnol Bioeng 55:1–10
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/orchitosan. Chemosphere 54:951–967
Chowdhury S, Saha P (2010) Sea shell powder as a new adsorbent to remove Basic Green 4 (Malachite Green) from aqueous solutions: equilibrium, kinetic and thermodynamic studies. Chem Eng J 164:168–177
Chowdhury S, Saha PD (2011) Biosorption kinetics, thermodynamics and isosteric heat of sorption of Cu(II) onto Tamarindus indica seed powder. Colloids Surf B Biointerfaces 88:697–705
Dubinin MM (1960) The potential theory of adsorption of gases and vapours for adsorbents with energetically non-uniform surface. Chem Rev 60:235–266
Garg VK, Gupta R, Yadav AB, Kumar R (2003) Dye removal from aqueous solution by adsorption on treated sawdust. Bioresour Technol 89:121–124
Gupta VK, Jain CK, Ali I, Sharma M, Saini VK (2003) Removal of cadmium and nickel from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interface Sci 271(2):321–328
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant pattern conditions. Ind Eng Chem Fundam 5:212–223
Hasar H (2003) Adsorption of nickel (II) from aqueous solution onto activated carbon prepared from almond husk. J Hazard Mater B97:49–57
Ho YS, McKay G (1998) A comparison of chemisorptions kinetic models applied to pollutant removal on various sorbents. Trans IChemE 76B:332–340
Huang LH, Sun YY, Yang T, Li L (2010) Adsorption behavior of Ni (II) on lotus stalks derived active carbon by phosphoric acid activation. Desalination 268:12–19
Jayaram K, Prasad MNV (2009) Removal of Pb(II) from aqueous solution by seed powder of Prosopis juliflora DC. J Hazard Mater 169:991–997
Kannan N, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—a comparative study. Dyes Pigments 51(1):25–40
Kelleher BP, O’Callaghan MN, Leahy MJ, O’Dwyer TF, Leahy JJ (2002) The use of fly ash from the combustion of poultry litter for the adsorption of chromium(III) from aqueous solution. J Chem Technol Biotechnol 77:1212–1218
Kumar PS, Ramalingam S, Sathyaselvabala V, Kirupha SD, Murugesan A, Sivanesan S (2012) Removal of cadmium(II) from aqueous solution by agricultural waste cashew nut shell. Korean J Chem Eng 29(6):756–768
Lalhruaitluanga H, Prasad MNV, Radha K (2011) Potential of chemically activated and raw charcoals of Melocanna baccifera for removal of Ni(II) and Zn(II) from aqueous solutions. Desalination 271:301–308
Lawal OS, Sanni AR, Ajayi IA, Rabiu OO (2010) Equilibrium, thermodynamic and kinetic studies for the biosorption of aqueous lead(II) ions onto the seed husk of Calophyllum inophyllum. J Hazard Mater 177:829–835
Momčilović M, Purenović M, Bojić A, Zarubica A, Ranđelović M (2011) Removal of lead(II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination 276:53–59
Mondal MK, Singh RS, Kumar A, Prasad BM (2011) Removal of acid red-94 from aqueous solution using sugar cane dust: an agro-industry waste. Korean J Chem Eng 28(6):1386–1392
Okoye AI, Ejikeme PM, Onukwuli OD (2010) Lead removal from wastewater using fluted pumpkin seed shell activated carbon: adsorption modeling and kinetics. Int J Environ Sci Tech 7(4):793–800
Oliver MA (1997) Soil and human health: a review. Eur J Soil Sci 48:573–592
Petruzzelli D, Pagano M, Tiravanti G, Passino R (1999) Lead removal and recovery from battery wastewaters by natural zeolite clinoptilolite. Solvent Extr Ion Exch 17(3):677–694
Pons MP, Fuste CM (1993) Uranium uptake by immobilized cells of Pseudomonas strain EPS5028. Appl Microbiol Biotechnol 39:661–665
Raji C, Anirudhan TS (1998) Batch Cr(VI) removal by polyacrylamide-grafted sawdust: kinetics and thermodynamics. Water Res 32:3772–3780
Remoudaki E, Hatzikioseyian A, Kousi P, Tsezos M (2003) The mechanism of metals precipitation by biologically generated alkalinity in biofilm reactors. Water Res 37(16):3843–3854
Rivera-Utrilla J, Bautista-Toledo I, Ferro-Garcy’a MA, Moreno-Castilla C (2001) Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J Chem Technol Biotechnol 76:1209–1215
Saha P, Chowdhury S, Gupta S, Kumar I (2010) Insight into biosorption equilibrium, kinetics and thermodynamics of malachite green onto clayey soil of Indian origin. Chem Eng J 165:874–882
Sharma YC, Prasad G, Rupainwar DC (1991) Removal of Ni(II) from aqueous solutions by sorption. Int J Environ Stud 37:183–191
Singh J, Ali A (2012) Kinetics, thermodynamics and breakthrough studies of biosorption of Cr(VI) using Arachis hypogea shell powder. Res J Chem Environ 16:69–79
US Environmental Protection Agency (USEPA) (2006) Edition of the drinking water standards and health advisories, summer, EPA, 822-R-06-013
Vinod VTP, Sashidhar RB, Sivaprasad N, Sarma VUM, Satyanarayana N, Kumaresan R, Rao TN, Raviprasad P (2011) Bioremediation of mercury (II) from aqueous solution by gum karaya (Sterculia urens): a natural hydrocolloid. Desalination 272:270–277
Wang XS, Qin Y (2005) Equilibrium sorption isotherms for of Cu2+ on rice bran. Process Biochem 40:677–680
Yan G, Viraraghavan T (2001) Heavy metal removal in a biosorption column by immobilized M. rouxii biomass. Bioresour Technol 78:243–249
Zhao G, Li M, Hu Z, Hu H (2005) Dissociation and removal of complex chromium ions containing in dye wastewaters. Sep Purif Technol 43(3):227–232
Acknowledgments
Authors acknowledge UGC, New Delhi, for financial support (Ref. No. F1-17.1/20/01 MANF. SIK-HAR-6208/SAIII website), IMTECH Chandigarh for providing AAS facility and M.M.E.C., M.M.U., Mullana, Ambala, for providing the other research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kaur, R., Singh, J., Khare, R. et al. Batch sorption dynamics, kinetics and equilibrium studies of Cr(VI), Ni(II) and Cu(II) from aqueous phase using agricultural residues. Appl Water Sci 3, 207–218 (2013). https://doi.org/10.1007/s13201-012-0073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0073-y