Abstract
To determine factors influencing failure-to-rescue in patients with complications following cytoreductive surgery and HIPEC. A retrospective analysis of patients enrolled in the Indian HIPEC registry was performed. Complications were graded according to the CTCAE classification version 4.3. The 30- and 90-day morbidity were both recorded. Three hundred seventy-eight patients undergoing CRS with/without HIPEC for peritoneal metastases from various primary sites, between January 2013 and December 2017 were included. The median PCI was 11 [range 0–39] and a CC-0/1 resection was achieved in 353 (93.5%). Grade 3–4 morbidity was seen 95 (25.1%) at 30 days and 122 (32.5%) at 90 days. The most common complications were pulmonary complications (6.8%), neutropenia (3.7%), systemic sepsis (3.4%), anastomotic leaks (1.5%), and spontaneous bowel perforations (1.3%). Twenty-five (6.6%) patients died within 90 days of surgery due to complications. The failure-to-rescue rate was 20.4%. Pulmonary complications (p = 0.03), systemic sepsis (p < 0.001), spontaneous bowel perforations (p < 0.001) and PCI > 20 (p = 0.002) increased the risk of failure-to-rescue. The independent predictors were spontaneous bowel perforation (p = 0.05) and systemic sepsis (p = 0.001) and PCI > 20 (p = 0.02). The primary tumor site did not have an impact on the FTR rate (p = 0.09) or on the grade 3–4 morbidity (p = 0.08). Nearly one-fifth of the patients who developed complications succumbed to them. Systemic sepsis, spontaneous bowel perforations, and pulmonary complications increased the risk of FTR and multidisciplinary teams should develop protocols to prevent, identify, and effectively treat such complications. All surgeons pursuing this specialty should perform a regular audit of their results, irrespective of their experience.
Similar content being viewed by others
Introduction
In the recent past, peritoneal cancer dissemination was perceived as a disease process worth only “palliation.” The dual treatment modality of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has provided an opportunity for longer survival and even cure in some patients. However, there remains hesitancy in subjecting a patient who has just undergone an aggressive local surgical treatment, to heated chemotherapy, which carries its own morbidity. It is a given that as complexities of surgical procedures increase, the risk of complications and therefore risk of perioperative morbidity and mortality increases in proportion.
CRS and HIPEC has reported mortality rates of 0–17% and morbidity rates of 0–52% [1,2,3]. In most studies, hospital mortality and the 30-day or 90-day morbidity have been used as parameters to evaluate surgical quality. Recently, failure-to-rescue (FTR) has emerged as a yard stick for evaluation of surgical quality and is defined as “death in a patient due to one or more complications after surgery.” [4]. This concept was first proposed as a hospital quality metric by Silber et al. to explain varying mortality rates following surgery [5]. For any surgical procedure, it is not just the surgical skill and clinical acumen but the nature of the surgery itself and the underlying patient and disease related factors that lead to morbidity. Hence, any surgical procedure will be lead to complications irrespective of the surgeon’s experience [6]. But with experience, the proportion of patients dying due to these complications reduces [7]. In our experience, the surgical morbidity has been at par with published reports from expert centers; however, the failure-to-rescue (FTR) rate was high [8]. We carried out this study to determine the factors responsible for FTR in Indian patients undergoing CRS and HIPEC.
Materials and Methods
The Indian network for development of peritoneal surface oncology (INDEPSO) is an independent collaborative group of Indian surgeons specializing in the management of peritoneal surface malignancy (PM). All patients enrolled by members of INDEPSO into the Indian HIPEC registry since its inception to December 2017 were included in this retrospective study. Patients undergoing CRS ± HIPEC for gastrointestinal, gynecological, and other rare peritoneal metastases were included. The goal of surgery was to obtain a CC-0 resection. Patients with an Eastern Cooperative oncology group (ECOG) performance status of 0/1, American Society of Anaesthesiologists (ASA) classes 1–3 and without any distant unresectable metastases were taken up for surgery. For patients with an albumin level of less than 3 g/dl, nutritional supplementation is provided or surgery is deferred. Intensive spirometry and other exercises to improve pulmonary capacity are started 7–15 days prior to the planned procedure. Some surgeons routinely administer intravenous fluids at 1 ml/kg/h, starting 12–24 h prior to surgery to patients scheduled for HIPEC with cisplatin or oxaliplatin, others do not. Each center has its own protocol for intra and post-operative management. At some centers, all patients undergoing diaphragmatic surgery receive post-operative ventilation, at others, they do not. HIPEC is performed by the open, semi-open, or closed method using the recommended drug regimens for each indication [9]. Two surgeons performed early post-operative intraperitoneal chemotherapy (EPIC) either alone or in combinations with HIPEC. EPIC is performed with paclitaxel 25 mg/m2 in 1 l of 1.5% peritoneal dialysis fluid infused daily for 5 days [10].
The perioperative outcomes were evaluated and complications were graded according to the CTCAE classification version 4.3 [11]. The 90-day morbidity and mortality were both recorded. The complications that failed to resolve within 30 days of surgery or new complications that developed after 30 days were included in the 90-day morbidity. Grade 3–4 complications were considered “major morbidity.” Failure-to-rescue (FTR) rate was defined as the percentage of patients with complications following surgery, dying due to complications. In the hospital, mortality was defined as all-cause mortality occurring within 30 days of surgery or till discharge from the hospital, whichever is later. Patients dying after discharge due to unknown causes were excluded from the FTR analysis.
Categorical data were compared using the ×2 test and Fisher’s exact test and Mann-Whitney U test was used for continuous variables. Clinically relevant variables, as well as variables with a p value < 0.15 on univariate analysis, were evaluated in multivariable logistic regression model. The model was selected using a forward stepwise method. All statistical tests were two-sided, and the significance level was 0.05. SPSS Version 20 (SPSS Inc., Chicago, IL) and MedCalc Version 12.2 were used for analysis.
Results
From January 2013 to December 2017, 378 patients were enrolled in the registry by 12 surgeons. Forty-eight (12.6%) were over the age of 65 years. Two hundred ninety-two (77.3%) were females. The primary tumor site was ovary in 142 (37.5%), appendix in 106 (28.0%), colorectal in 66 (17.4%), mesothelioma in 23 (6.0%), and uncommon primary sites in 42 (11.1%). The median PCI was 11 [range 0–39] and 131 (34.6%) had a PCI > 20. A CC-0/1 resection was achieved in 353 (93.3%). HIPEC was performed in 301 (80%) patients, EPIC in 32 (8.4%) (4 had HIPEC and EPIC both) and no intraperitoneal chemotherapy (IPC) was administered in 38 (11.5%). The HIPEC drug regimen comprised of cisplatin in 129 (41.3%), mitomycin-C in 122 (39.1%), and oxaliplatin in 39 (12.5%). Resection of 4 or more organs was performed in 142 (37.6%) patients and 2 or more bowel anastomosis in 150 (39.7%). The median ICU stay was 2 days and the hospital stay was 15 days [range 5–69 days].
Morbidity and Mortality
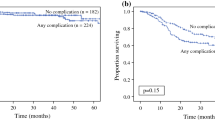
Grade 3–4 morbidity was seen in 95 (25.1%) patients at 30 days and 122 (32.5%) at 90 days. In 19 patients, the complications failed to resolve within 30 days and in 8 others new complications developed after 30 days of surgery. Twenty-two patients had more than 1 major complication. Twenty (5.2%) patients died within 30 days and 25 (6.6%) within 90 days of surgery due to complications. 5/25 patients (20%) died more than 30 days after surgery. The failure-to-rescue rate was 20.4%. A comparison of patients with and without complications is provided in Table 1.
In the group with complications, the proportion of patients with a PCI > 20 (p = 0.02), 4 or more organ resections (p = 0.065), 2 or more bowel anastomosis (p = 0.08), and those with a duration of surgery > 8 h (p < 0.0001) was higher. The commonest complications (grade 3–4) were pulmonary in 26 (6.8%), neutropenia in 15 (3.9%), systemic sepsis in 14 (3.7%), wound dehiscence in 9 (2.3%), cardiac in 7 (1.8%), urinary fistula in 13 (3.4%), anastomotic leak in 12 (3.1%), spontaneous bowel perforations in 5 (1.3%), and others in 12 (3.1%). Renal complications occurred in 5 (1.3%) patients. The pulmonary complications included pneumonitis requiring return to ICU and/or reintubation, acute respiratory distress syndrome, pleural effusion, and hydropneumothorax. Fourteen patients (11.4%) underwent reoperation for management of complications and 10 (8.1%) were readmitted following discharge for the same. Prior chemotherapy (p = 0.03), PCI > 20 (p = 0.02) and resection of 4 or more organs (p = 0.019) were independent predictors of a higher incidence of complications. There was a trend towards increased morbidity in patients who received HIPEC (p = 0.06). The factors affecting major morbidity are detailed in supplementary section 1. The only independent predictor of a higher mortality was systemic sepsis (p = 0.001). Though the group of patients with no complications had a higher proportion of ovarian cancer patients (p < 0.001), the primary tumor site was not a predictor of a higher risk of complications.
Failure-to-Rescue
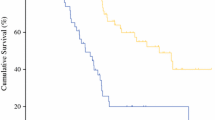
Of the 122 patients who developed grade 3–4 complications within 90 days, 25 (20.4%) died due to these complications, i.e., 20.4% could not be rescued. The FTR rate was different for each complication (Fig. 1). A comparison between patients who were rescued and not rescued is provided in Table 2.
None of the patients with cardiac complications or anastomotic leaks died due to them. However, 64.5% of the patients with systemic sepsis and 60% of the patients with spontaneous bowel perforations died because of the respective complication. A higher rate of FTR was observed in patients with PCI > 20 (p = 0.02), more than one bowel anastomosis (p = 0.037), pulmonary complications (p = 0.03), spontaneous bowel perforation (p = 0.005), and systemic sepsis (p = 0.001) (Table 3). The two independent predictors were spontaneous bowel perforation (p = 0.05) and systemic sepsis (p = 0.001) (Table 4). The failure-to-rescue rate did not depend on the age (p = 0.29) or sex (p = 0.39), number of organs resected (p = 0.15), number of bowel anastomosis (p = 0.33), or when surgery was performed before 2014 (p = 0.32). The primary tumor site did not have an impact on the FTR rate (p = 0.09).
Discussion
The overall 90-day grade 3–4 morbidity of 32.5% in this study can be considered acceptable but the mortality of 6.6% is high compared to current published reports [12, 13]. Similarly, the FTR rate (25.4%) is significantly higher than published reports [14, 15]. Even in their early experience, only 9.02% patients with complications could not be rescued at hospital Lyon Sud [14]. It has been shown that FTR depends on the type of complication. In Passot’s study, renal failure was identified as a factor predicting FTR. Li et al. concluded that ASA class 4, major morbidity, and dependent functional status were independent predictors of FTR [14, 15]. Kim et al. showed that pneumonia, intestinal fistula, and pancreatic leaks had a higher probability of mortality [16].
In this study, systemic sepsis, spontaneous bowel perforations, and pulmonary complications were associated with a high FTR rate. Spontaneous bowel perforations are perforations that occur in regions of the bowel where no anastomosis/suturing has been performed. These may be probably due to unnoticed iatrogenic injury to the bowel either from an energy source used during surgery or during HIPEC when it is performed by the closed method. The second concern is systemic sepsis. 3.7% patients had systemic sepsis and though we have not captured this information in the registry for all patients, most of these infections which resulted in failure-to-rescue were due to multidrug-resistant organisms (MDR) like carbapenem-resistant enterococci (CRE), vancomycin-resistant enterococci (VRE), and methicillin-resistant Staphylococcus aureus (MRSA) [17]. Patients undergoing extensive surgery with IPC leading to an immunocompromised state are at an increased risk of sepsis especially with MDR organisms [17]. All patients who died due to bowel perforations had developed systemic sepsis. In contrast, none of the patients with anastomotic leaks died due to it. We attribute this difference to the development of sepsis in patients with perforations. Even if safe surgical practices and infection control measures are followed, patients may harbor organisms in indwelling devices like chemo-ports. The problem of systemic sepsis due to MDR organisms is a community problem in the country due to injudicious use of antibiotics which lead to the growth of drug-resistant organisms and cannot completely be avoided even if all precautions are taken. At one institution, all patients are screened for MDR organisms with a throat and stool culture and antibiotics are given accordingly before surgery [18]. This calls for a judicious use of IPC. Use of IPC was associated with a higher incidence of complications (p = 0.02). The risk of sepsis increases in presence of any complication. Where there is a doubt regarding the added benefit of IPC, it should be avoided especially if the risk of morbidity is high.
Thirdly, every fifth patient with a pulmonary complication died because of it. Extensive upper abdominal surgery specifically diaphragm stripping has been associated with an increased incidence of pulmonary complications by some investigators and not by others [19, 20]. In our study, since majority of the patients had upper abdominal surgery, we were not able to demonstrate the impact on pulmonary complications. With changing life styles and dietary patterns, abdominal obesity with or without general obesity has become common in urban Indians [21, 22]. The ECOG performance status and ASA grade do not take into account the physical capacity. Even in patients who are not morbidly obese, but have abdominal obesity and do not perform regular physical exercise, the post-operative pulmonary function can be compromised. A pre-operative pulmonary function test that will usually be normal is such patients or show a restrictive pattern related to poor effort. Most Indian surgeons are cautious in selecting older patients for surgery for these reasons. Only 12.6% of our patients were aged over 65 though studies have shown good results over the age of 70 in Caucasians [23]. At one center, non-invasive ventilation is used post-operatively for all patients to aid recovery of lung function [24]. Though we have not performed anthropometric measurements and calculated BMI, this seems to be the most plausible explanation for increased pulmonary complications in patients not at an increased baseline risk and should be prospectively evaluated. In the western population, obesity has not been associated with increased morbidity [25, 26].
There is another group of patients who have extensive disease and/or present with gross ascites. Optimizing pulmonary function is difficult in such patients and the risk for pulmonary complications is increased. Extensive surgery leads to prolonged ventilation and ventilator acquired pneumonia in these patients leading to FTR.
In our study, the proportion of complications was higher in patients with mesothelioma and lower in those with ovarian cancer. The increase in complications due to HIPEC may be due to surgeons being on the learning curve and inclusion of various primary tumor sites. Only few patients had albumin levels of < 3 g/dL since most surgeons used this as an exclusion criterion. Hence, we are not able to evaluate its impact on complications. The administration of chemotherapy before surgery and multi-visceral resections, known as risk factors for complications, increased the risk of complications in our patients [27, 28]. The number of resected organs is related not only to disease extent, but also to the tumor location in anatomic sites where a conservative approach is technically not feasible. Thus, it is not always possible to prevent complications but mortality can be decreased if complications are identified early and treated in a timely manner [29, 30]. The benefit of this approach has been demonstrated at Lyon where after the introduction of a “clinical pathway” for perioperative care, FTR reduced from 9.02% of 1.02% (p < 0.001) [14]. All patients are shifted from the intensive care unit to a step down unit where continuous cardiorespiratory monitoring is performed till deemed suitable to be managed in the ward. A dedicated nurse takes care of counseling of patients and families during the perioperative period, facilitating communication between patients and physicians. Some of the centers contributing to this study have developed such protocols [14].
The importance of using the appropriate classification for complications is demonstrated. In the Clavien-Dindo classification, systemic toxicity is not reported which is common in patients undergoing HIPEC and can lead to underreporting of complications by nearly 50% as shown in one study [31]. The National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) classification includes both surgical and medical morbidity and is preferred by most experts to report the morbidity of CRS and HIPEC [32]. Secondly, in many patients, complications do not resolve within 30 days and new complications develop after 30 days. In our experience, nearly 20% complications did not resolve within 30 days and 20% deaths occurred after 30 days. Thus, as proposed by Alyami et al., the 90-day morbidity using the NCI-CTCAE classification should be used to report the morbidity of CRS and HIPEC [33]. This means that perioperative care should also continue after discharge from hospital. Patients should be aware that complications can arise after discharge, the warning signs of expected complications and should report to a suitable medical facility for proper diagnosis and management.
There are several limitations to this study. First, this was a retrospective study utilizing data from a registry. The indications, experience of surgeons, and perioperative management strategies are not uniform at centers. We made a comparison between outcomes at different centers (public and private both) in nearly the same patient population in a prior publication and found no difference in morbidity and mortality [8]. Secondly, we failed to capture the causative organisms for sepsis. This is one limitation of our method of data capturing—it is not possible to capture many details and we are dependent on surgeons and hospital records for providing the information when a particular study is performed.
The morbidity and mortality from CRS and HIPEC can be high even at centers that have crossed the peak of the learning curve [34]. Though the FTR rate reflects the performance of the multidisciplinary team, yet, the responsibility of the surgeon/surgical team is not undermined but is increased. The surgeon can falter in patient selection, improper preparation for surgery, embarking on surgery without a proper surgical plan, misinterpretation of imaging findings, intraoperative injuries, failure to identify the complication early, improper management of complications, and improper communication with other members of the multidisciplinary team. Hence, surgeons need to put in a lot more effort and take a lead in developing multi-disciplinary expertise at their respective centers. A pulmonologist should be part of such a team. A comparison with results obtained at the most experienced center in the world may not be considered ideal since this is a multi-centric retrospective study representing the learning phase of centers in a country where patients are bearing the cost of the procedure often leading to financial limitations and patients are genetically different. Yet, this comparison shows us what best can be achieved and whilst we are formulating treatment guidelines based on western results, we must also aim to achieve their treatment results. It is an eye-opener for developing common protocols specific to countries to improve outcomes.
As the popularity of this procedure and the number of centers offering it increases, the number of surgeons evaluating their results not just in terms of morbidity and FTR but also other factors like rates of complete cytoreduction and oncological outcomes (early recurrence) should increase.
Conclusions
Nearly one-fifth of the patients who developed complications following CRS with/without HIPEC succumbed to them. Systemic sepsis, spontaneous bowel perforations, and pulmonary complications were independently predictors of increased rate of FTR and multidisciplinary teams should develop protocols to prevent, identify and effectively treat such complications. All surgeons pursuing this specialty should perform a regular audit of their results, irrespective of their experience.
References
Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D, French Surgical Association (2010) Toward curative treatment of peritoneal carcinomatosis from non-ovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 116:5608–5618
Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gómez Portilla A, de Hingh IHJT, Ceelen WP, Pelz JO, Piso P, González-Moreno S, van der Speeten K, Morris DL (2012) Early and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30:2449–2456
Bakrin N, Bereder JM, Decullier E, Classe JM, Msika S, Lorimier G, Abboud K, Meeus P, Ferron G, Quenet F, Marchal F, Gouy S, Morice P, Pomel C, Pocard M, Guyon F, Porcheron J, Glehen O, FROGHI (FRench Oncologic and Gynecologic HIPEC) Group (2013) Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 39:1435–1443
Silber J, Romano P, Rosen A, Wang Y, Even-Shoshan O, Volpp K (2007) Failure-to-rescue: comparing definitions to measure quality of care. Med Care 45:918e925
Silber JH, Williams SV, Krakauer H, Schwartz JS (1992) Hospital and patient characteristics associated with death after surgery: a study of adverse occurrence and failure to rescue. Med Care 30:615–629
Mercier F, Cotte E, Glehen O, Passot G (2017) Why morbidity is not an adequate metric for evaluation of surgical quality. Ann Surg. https://doi.org/10.1097/SLA.0000000000002256
Passot G, Vaudoyer D, Villeneuve L, Kepenekian V, Beaujard AC, Bakrin N, Cotte E, Gilly FN, Glehen O (2016) What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J Surg Oncol 113(7):796–803. https://doi.org/10.1002/jso.24248
Bhatt A, Mehta S, Zaveri S, Rajan F, Ray M, Sethna K, Katdare N et al (2018) Treading the beaten path with old and new obstacles: a report from the Indian HIPEC registry. Int J Hyperth:1–9. https://doi.org/10.1080/02656736.2018.1503345
Van der Speeten K, Lemoine L (2018) HIPEC methodology, comparison of techniques, and drug regimens: is there a need for standardization? In: Management of Peritoneal Metastases- Cytoreductive Surgery, HIPEC and Beyond. Springer, pp 298–391
Douchy T, Lemoine L, Van der Speeten K (2018) Early postoperative intraperitoneal chemotherapy: current role and future perspectives. In: Management of Peritoneal Metastases- Cytoreductive Surgery, HIPEC and Beyond. Springer, pp 392–511
United States Department of Public Health and Human Services, NIH, NCI: Common Toxicity Criteria for Adverse Events (CTCAE). National Cancer Institute, June 2010. http://evs.nci.nih.gov/ftp1/CTCAE_Vers._4.03_2010-06- 14;_QuickReference_5x7.pdf
Baumgartner JM, Kwong TG, Ma GL, Messer K, Kelly KJ, Lowy AM (2016) A novel tool for predicting major complications after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 23(5):1609–1617. https://doi.org/10.1245/s10434-015-5012-3
Moran B, Cecil T, Chandrakumaran K, Arnold S, Mohamed F, Venkatasubramaniam A (2015) The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Color Dis 17:772–778
Passot G, Vaudoyer D, Villeneuve L, Wallet F, Beaujard AC, Boschetti G, Rousset P, Bakrin N, Cotte E, Glehen O (2017) A perioperative clinical pathway can dramatically reduce failure-to-rescue rates after cytoreductive surgery for peritoneal carcinomatosis: a retrospective study of 666 consecutive cytoreductions. Ann Surg 265(4):806–813
Li KY, Mokdad AA, Minter RM, Mansour JC, Choti MA, Augustine MM, Polanco PM (2017) Failure to rescue following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Res 214:209–215. https://doi.org/10.1016/j.jss.2017.02.048
Kim B, Alzharani N, Valle S, Liauw W, Morris D (2017) Treatment-related post-operative mortality after cytoreductive surgery and perioperative intraperitoneal chemotherapy. J Peritoneum (and other serosal surfaces) 2:65
Gupta V, Singla N, Gombar S, Palta S, Chander J (2018) Prevalence of multidrug-resistant pathogens and their antibiotic susceptibility pattern from late-onset ventilator-associated pneumonia patients from a tertiary-care hospital in North India. J Assoc Chest Phys 6:4–11
Laxminarayan R, Chaudhury RR (2016) Antibiotic resistance in India: drivers and opportunities for action. PLoS Med 13(3):e1001974. https://doi.org/10.1371/journal.pmed.1001974
Chéreau E, Ballester M, Selle F, Cortez A, Pomel C, Darai E, Rouzier R (2009) Pulmonary morbidity of diaphragmatic surgery for stage III/IV ovarian cancer. Int J Obstet Gynaecol 116(8):1062–1068
Franssen B, Tabrizian P, Weinberg A, Romanoff A, Tuvin D, Labow D, Sarpel U (2015) Outcome of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on patients with diaphragmatic involvement. Ann Surg Oncol 22:1639–1644
Gouda J, Prusty RK (2014) Overweight and obesity among women by economic stratum in urban India. J Health Popul Nutr 32(1):79–88
Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP, Dhandania VK, Madhu SV, Rao PV, Geetha L, Subashini R, Unnikrishnan R, Shukla DK, Kaur T, Mohan V, Das AK, ICMR-INDIAB Collaborative Study Group (2015) Prevalence of generalized & abdominal obesity in urban & rural India--the ICMR-INDIAB study (phase-I) [ICMR- NDIAB-3]. Indian J Med Res 142(2):139–150
Alyami M, Lundberg P, Kepenekian V, Goéré D, Bereder JM, Msika S, Lorimier G et al (2016) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis in the elderly: a case-controlled, multicenter study. Ann Surg Oncol 23(Suppl 5):737–745
Kammar P, Waghoo S, Anam J, Agarwal A, Borkar N, Mehta S, et al (2018) Utility of bipap (biphasic positive airway pressure) ventilation in crs+hipec (cytoreductive surgery+ hyperthermic intraperiotneal chemotherapy). Pleura and Peritoneum; 1, Special Suppl, pp sA6–sA463, September 2018 (page sA123)
McPartland SJ, Goodman MD (2014) The effect of elevated body mass index on outcomes following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 21:1463–1467
Votanopoulos KI, Swords DS, Swett KR, Randle RW, Shen P, Stewart JH, Levine EA (2013) Obesity and peritoneal surface disease: outcomes after cytoreductive surgery with hyperthermic intra- peritoneal chemotherapy for appendiceal and colon primary tumours. Ann Surg Oncol 20:3899–3904
Baratti D, Kusamura S, Mingrone E, Balestra MR, Laterza B, Deraco M (2012) Identification of a subgroup of patients at highest risk for complications after surgical cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg 256:334–341
Franko J, Gusani NJ, Holtzman MP, Ahrendt SA, Jones HL, Zeh HJ, Bartlett DL (2008) Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol 15(11):3065–3072
Johnston M, Arora S, Anderson O, King D, Behar N, Darzi A (2015) Escalation of care in surgery: a systematic risk assessment to prevent avoidable harm in hospitalized patients. Ann Surg 261:831–838
Johnston MJ, Arora S, King D, Bouras G, Almoudaris AM, Davis R, Darzi A (2015) A systematic review to identify the factors that affect failure to rescue and escalation of care in surgery. Surgery 157:752–763
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Youkan R, Kamara S, Buratti D et al (2008) Morbidity, toxicity, and mortality classification systems in the local regional treatment of peritoneal surface malignancy. J Surg Oncol 98:253–257
Alyami M, Kim BJ, Villeneuve L, Vaudoyer D, Képénékian V, Bakrin N, Gilly FN, Cotte E, Glehen O, Passot G (2018) Ninety-day post-operative morbidity and mortality using the National Cancer Institute’s common terminology criteria for adverse events better describe post-operative outcome after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperth 34(5):532–537. https://doi.org/10.1080/02656736.2017.1367846
Kusamura S, Baratti D, Deraco M (2012) Multidimensional analysis of the learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies. Ann Surg 255(2):348–356. https://doi.org/10.1097/SLA.0b013e3182436c28
Acknowledgments
The authors thank Professor Ramakrishnan Seshadri for giving his views on the subject and suggestions on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 63 kb)
Rights and permissions
About this article
Cite this article
Sinukumar, S., Mehta, S., Damodaran, D. et al. Failure-to-Rescue Following Cytoreductive Surgery with or Without HIPEC is Determined by the Type of Complication—a Retrospective Study by INDEPSO. Indian J Surg Oncol 10 (Suppl 1), 71–79 (2019). https://doi.org/10.1007/s13193-019-00877-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-019-00877-x