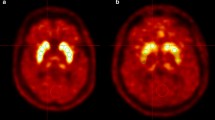
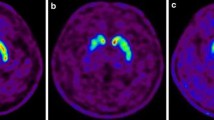
Abstract
Purpose
We present the findings of our final prospective study submitted to the U.S. Food and Drug Administration (FDA) for New Drug Application (NDA) approval for the use of 3,4-dihydroxy-6-[18F]fluoro-l-phenylalanine (F-18 FDOPA) positron emission tomography (PET) imaging for Parkinson’s disease (PD). The primary aim was to determine the sensitivity, specificity, and predictive values of F-18 FDOPA PET in parkinsonian patients with respect to clinical standard-of-truth (SOT). Secondary outcomes included the inter-rater reliability, and correlation of quantitative measures for PET with dopaminergic status.
Methods
In 68 parkinsonian subjects, F-18 FDOPA PET scan from 80 to 100 min was acquired following a CT scan. Scan images were presented to one expert in F-18 FDOPA image interpretation and two physicians with prior experience in I-123 FPCIT single-photon emission computed tomography image interpretation. Fifty-six subjects completed the study with a follow-up for SOT determination. Image readers were blind to the clinical/quantitative data; SOT clinician was blind to the image data.
Results
For 47 of the 56 patients, SOT was in agreement with the PET scan results. For nine patients, SOT suggested dopaminergic deficit, whereas the imaging showed normal uptake. The specificity and positive predictive values are 91% and 92%, respectively, suggesting high probability that those who test positive by the PET scan truly have dopaminergic degeneration. The sensitivity was 73%. Inter-rater agreement was 0.6–0.8 between the different readers.
Conclusion
Our prospective study demonstrates high specificity and moderate sensitivity of F-18 FDOPA PET for PD. We received NDA approval in October 2019.


Similar content being viewed by others
Data Availability
Contact the corresponding author for data requests.
References
Jennings DL, Seibyl JP, Oakes D, Eberly S, Murphy J, Marek K. (123I) beta-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in Parkinsonian syndrome: unmasking an early diagnosis. Arch Neurol. 2004;61:1224–9.
LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–19.
Chevalme YM, Montravers F, Vuillez JP, Zanca M, Fallais C, Oustrin J, et al. FDOPA-(18F): a PET radiopharmaceutical recently registered for diagnostic use in countries of the European Union. Braz Arch Biol Tech. 2007;50:77–90.
Eshuis SA, Jager PL, Maguire RP, Jonkman S, Dierckx RA, Leenders KL. Direct comparison of FP-CIT SPECT and F-DOPA PET in patients with Parkinson’s disease and healthy controls. Eur J Nucl Med Mol Imaging. 2009;36:454–62.
Calabria FF, Calabria E, Gangemi V, Cascini GL. Current status and future challenges of brain imaging with (18)F-DOPA PET for movement disorders. Hell J Nucl Med. 19:33–41.
Dhawan V, Ma Y, Pillai V, Spetsieris P, Chaly T, Belakhlef A, et al. Comparative analysis of striatal FDOPA uptake in Parkinson’s disease: ratio method versus graphical approach. J Nucl Med. 2002;43:1324–30.
Jokinen P, Helenius H, Rauhala E, Brück A, Eskola O, Rinne JO. Simple ratio analysis of 18F-fluorodopa uptake in striatal subregions separates patients with early Parkinson disease from healthy controls. J Nucl Med. 2009;50:893–9.
Snow BJ, Tooyama I, McGeer EG, Yamada T, Calne DB, Takahashi H, et al. Human positron emission tomographic [18F]fluorodopa studies correlate with dopamine cell counts and levels. Ann Neurol. 1993;34:324–30.
Morbelli S, Esposito G, Arbizu J, Barthel H, Boellaard R, Bohnen NI, et al. EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur J Nucl Med Mol Imaging. 2020;47:1885–912.
Center for Drug Evaluation and Research. NDA/BLA multi-disciplinary review and evaluation. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/200655Orig1s000MultidisciplineR.pdf.
Vernaleken I, Kumakura Y, Cumming P, Buchholz HG, Siessmeier T, Stoeter P, et al. Modulation of [18F]fluorodopa (FDOPA) kinetics in the brain of healthy volunteers after acute haloperidol challenge. NeuroImage. 2006;30:1332–9.
Gründer G, Vernaleken I, Müller MJ, Davids E, Heydari N, Buchholz HG, et al. Subchronic haloperidol downregulates dopamine synthesis capacity in the brain of schizophrenic patients in vivo. Neuropsychopharmacology. 2003;28:787–94.
Ruottinen HM, Rinne JO, Oikonen VJ, Bergman JR, Haaparanta MT, Solin OH, et al. Striatal 6-[18F]fluorodopa accumulation after combined inhibition of peripheral catechol-O-methyltransferase and monoamine oxidase type B: differing response in relation to presynaptic dopaminergic dysfunction. Synapse. 1997;27:336–46.
DeJesus OT, Shelton SE, Roberts AD, Nickles RJ, Holden JE. Effect of tetrabenazine on the striatal uptake of exogenous L-DOPA in vivo: a PET study in young and aged rhesus monkeys. Synapse. 2002;44:246–51.
Conway CR, Chibnall JT, Cumming P, Mintun MA, Gebara MAI, Perantie DC, et al. Antidepressant response to aripiprazole augmentation associated with enhanced FDOPA utilization in striatum: a preliminary PET study. Psychiatry Res. 2014;221:231–9.
Whone AL, Bailey DL, Remy P, Pavese N, Brooks DJ. A technique for standardized central analysis of 6-18 F-fluoro-L-DOPA PET data from a multicenter study. J Nucl Med. 2004;45:1135–45.
Reynés-Llompart G, Gámez-Cenzano C, Romero-Zayas I, Rodríguez-Bel L, Vercher-Conejero JL, Martí-Climent JM. Performance characteristics of the whole-body discovery IQ PET/CT System. J Nucl Med. 2017;58:1155–61.
Robeson W, Dhawan V, Ma Y, Bjelke D, Margouleff C, Chaly T, et al. Radiation absorbed dose to the basal ganglia from dopamine transporter radioligand 18F-FPCIT. Biomed Res Int. 2014;2014:498072.
Catafau AM, Tolosa E, Laloux P, vander Borght T, van Zandijcke M, de Geeter F, et al. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov Disord. 2004;19:1175–82.
Felicio AC, Godeiro-Junior C, Shih MC, Borges V, Silva SMA, Aguiar P de C, et al. Evaluation of patients with clinically unclear Parkinsonian syndromes submitted to brain SPECT imaging using the technetium-99m labeled tracer TRODAT-1. J Neurol Sci. 2010;291:64–8.
Oh M, Lee N, Kim C, Son HJ, Sung C, Oh SJ, et al. Diagnostic accuracy of dual-phase 18 F-FP-CIT PET imaging for detection and differential diagnosis of Parkinsonism. Sci Rep. 2021;11.
Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–12.
Acknowledgments
Special thanks to Mr. Claude Margouleff and Ms. Limei Zhuo for technical assistance with the PET studies.
Funding
This research was supported by funds from the Department of Radiochemistry/Cyclotron directed by Thomas Chaly. He is the senior author and had a role in the study design, data analysis, interpretation and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
The study was designed by Vijay Dhawan, Martin H Niethammer, Matthew Hellman, David Eidelberg, and Thomas Chaly. Material preparation and data collection were performed by Vijay Dhawan, Martin H Niethammer, Matthew Hellman, Toni M Fitzpatrick, David Bjelke, Jaskirat Singh, Loreta M Quatarolo, and Yoon Young Choi. The data analysis was performed by Vijay Dhawan, Martin L Lesser, Karalyn N Pappas, David Bjelke, Alice Oh, and Thomas Chaly. Project administrative support was provided by Toni M Fitzpatrick, Loreta M Quatarolo, Yoon Young Choi, and Alice Oh. Supervision was provided by David Eidelberg. The first draft of the manuscript was written by Vijay Dhawan, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to participate
This study was performed with Institutional Review Board approval of the Feinstein Institutes for Medical Research, and in compliance with the HIPAA Privacy Rule. Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 2013 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
Competing Interests
Vijay Dhawan, Martin H Niethammer, Martin L Lesser, Karalyn N Pappas, Matthew Hellman, Toni M Fitzpatrick, David Bjelke, Jaskirat Singh, Loreta M Quatarolo, Yoon Young Choi, Alice Oh, David Eidelberg, and Thomas Chaly declare that they have no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 88 kb)
Appendix
Appendix
A Fluorodopa F-18 FDOPA PET scan has the unique capability to quantify striatal neuronal loss. This SOR quantification may be helpful as an additional tool to evaluate the scans. Even though we have used SOR quantification procedure in the past for routine clinical interpretation of the PET scan results, for this clinical study the SOR values were not used for the visual determination of the PET scan results. The SOR values were blinded to the image readers and the physician collecting SOT. This SOR quantification is straightforward and does not require any special software program (see below).
We calculated the SOR values for all patients to see how the PET scan results correlate with the SOR values to assess loss of dopaminergic neurons. If the SOR values can be used to further confirm the visual interpretation of the PET scan results, it may be used as a supportive tool for the differential diagnosis of Parkinsonian syndromes. The average values of the left posterior putamen for the positive scans are 1.790 compared to the average values of the left posterior putamen for the negative scans is 2.627. The negative scans are normal, and the values agree with the SOR values for normal subjects (2.545) from our previously unpublished retroactive study (study #17). L = left; R = right.
We did not use ipsilateral/contralateral classification because the images were only read visually without any additional quantification or clinically relevant data. Moreover, instead of focusing on the contralateral posterior putamen (the site of early-stage dopaminergic deficit) this also represents the worst-case scenario for image interpretation.
SOR estimation:
Even though we have an automated MRI-supported ROI selection methodology for F-18 FDOPA PET data analysis available, for this study we used our manual method that is in use for more than 20 years that we find very straightforward and easy to use and does not require any special software except what is supplied by the PET scanner manufacturers for image processing, thereby making it easy to implement anywhere.
Region-of-interest (ROI) analysis is carried out on the F-18 FDOPA PET images by first adding the central five slices on which the striatum is visible (usually 8–9 slices on most scanners; composite slice thickness is 16–18 mm). Large circular ROIs (20 cm2) are placed on both left and right occipital lobes. The average count value is calculated and then the whole image is divided by this value to generate the striatal/occipital ratio (SOR) image. Four small circular ROIs (1.2 cm2) are placed on the transaxial slice along the axis of the striatum covering the caudate and anterior, middle, and posterior sections of the putamen. The ratio values are then compared with the values obtained from a database of normal control subjects. Anything less than two standard deviations below the normal value is considered abnormal
Distribution of striatal/occipital ratio (SOR) values for the 56 subjects with F-18 FDOPA PET imaging and SOT data in the right (left panel) and left (right panel) posterior putamen.
Rights and permissions
About this article
Cite this article
Dhawan, V., Niethammer, M.H., Lesser, M.L. et al. Prospective F-18 FDOPA PET Imaging Study in Human PD. Nucl Med Mol Imaging 56, 147–157 (2022). https://doi.org/10.1007/s13139-022-00748-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-022-00748-4