Abstract
Purpose
The objective of this study was to describe to develop methods of rodent leukocyte isolation and radiolabeling for in vivo inflammation imaging.
Methods
Thigh muscle inflammation was induced by injection of collagenase. Blood was collected from the jugular vein and separated by Histopaque. The collected cells were incubated in a 37 °C CO2 incubator for 1~2 h. After incubation, 99mTc-HMPAO and 18F-FDG were used to treat leukocytes followed by incubation for 30 min. 99mTc-HMPAO and 18F-FDG labeled autologous leukocytes were injected into the tail veins of rats. The images were then acquired at various time points. Image-based lesion to normal muscle ratio was compared.
Results
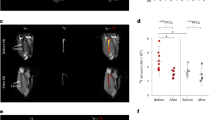
After Histopaque separation, the proportion of lymphocytes was higher than that of other cell types. After CO2 incubation, the collected leukocytes were viable, while room temperature exposed leukocytes without CO2 incubation were non-viable. Granulocytes, especially, were more quickly influenced by various conditions than the mononuclear cells. Labeling efficiencies of 99mTc-HMPAO and 18F-FDG were 4.00 ± 2.06 and 1.8%, respectively. 99mTc-HMPAO- and 18F-FDG-labeled leukocytes targeted well the inflamed lesion. 99mTc-HMPAO-labeled leukocytes, but not 18F-FDG-labeled leukocytes, were found in the abdomen activity.
Conclusion
Inflamed lesions of rats were well visualized using autologous radiolabeled leukocytes. This method might provide good information for understanding inflammatory diseases.







Similar content being viewed by others
References
Pellico J, Lechuga-Vieco AV, Almarza E, Hidalgo A, Mesa-Nuñez C, Fernández-Barahona I, et al. In vivo imaging of lung inflammation with neutrophil-specific 68Ga nano-radiotracer. Sci Rep. 2017;7:13242.
Velikyan I. Prospective of 68Ga radionuclide contribution to the development of imaging agents for infection and inflammation. Contrast Media Mol Imaging. 2018;9713691.
Caobelli F, Evangelista L, Quartuccio N, Familiari D, Altini C, Castello A, et al. Role of molecular imaging in the management of patients affected by inflammatory bowel disease: state-of-the-art. World J Radiol. 2016;8:829–45.
Tsopelas C. Radiotracers used for the scintigraphic detection of infection and inflammation. ScientificWorldJournal. 2015;676719.
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9:7204–18.
Serhan CN. Treating inflammation and infection in the 21stcentury: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31:1273–88.
Signore A, Glaudemans AW. The molecular imaging approach to image infections and inflammation by nuclear medicine techniques. Ann Nucl Med. 2011;25:681–700.
Hughes DK. Nuclear medicine and infection detection: the relative effectiveness of imaging with 111In-oxine-, 99mTc-HMPAO-, and 99mTc-stannous fluoride colloid-labeled leukocytes and with 67Ga-citrate. J Nucl Med Technol. 2003;31:196–201.
Salmanoglu E, Kim S, Thakur ML. Currently available radiopharmaceuticals for imaging infection and the holy grail. Semin Nucl Med. 2018;48:86–99.
Goldsmith SJ, Vallabhajosula S. Clinically proven radiopharmaceuticals for infection imaging: mechanisms and applications. Semin Nucl Med. 2009;39:2–10.
Auletta S, Galli F, Lauri C, Martinelli D, Santino I, Signore A. Imaging bacteria with radiolabelled quinolones, cephalosporins and siderophores for imaging infection: a systematic review. Clin Transl Imaging. 2016;4:229–52.
Kniess T, Laube M, Wüst F, Pietzsch J. Technetium-99m based small molecule radiopharmaceuticals and radiotracers targeting inflammation and infection. Dalton Trans. 2017;46:14435–51.
Auletta S, Riolo D, Varani M, Lauri C, Galli F, Signore A. Labelling and clinical performance of human leukocytes labelled with 99mTc-HMPAO using Leukokit® with Gelofusine versus Leukokit® with HES as sedimentation agent. Contrast Media Mol Imaging. 2019;4368342.
de Vries EF, Roca M, Jamar F, Israel O, Signore A. Guidelines for the labelling of leucocytes with (99m)Tc-HMPAO. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur J Nucl Med Mol Imaging. 2010;37:1235.
Dumarey N, Egrise D, Blocklet D, Stallenberg B, Remmelink M, del Marmol V, et al. Imaging infection with 18F-FDG-labeled leukocyte PET/CT: initial experience in 21 patients. J Nucl Med. 2006;47:625–32.
Osman S, Danpure HJ. The use of 2-[18F] fluoro-2-deoxy-D-glucose as a potential in vitro agent for labelling human granulocytes for clinical studies by positron emission tomography. Int J Rad Appl Instrum B. 1992;19:183–90.
Bhattacharya A, Kochhar R, Sharma S, Ray P, Kalra N, Khandelwal N, et al. PET/CT with 18F-FDG-labeled autologous leukocytes for the diagnosis of infected fluid collections in acute pancreatitis. J Nucl Med. 2014;55:1267–72.
Jamar F, Buscombe J, Chiti A, Christian PE, Delbeke D, Donohoe KJ, et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med. 2013;54:647–58.
Lawal I, Sathekge M. F-18 FDG PET/CT imaging of cardiac and vascular inflammation and infection. Br Med Bull. 2016;120:55–74.
Webb DR. Animal models of human disease: inflammation. Biochem Pharmacol. 2014;87:121–30.
Jiminez JA, Uwiera TC, Douglas Inglis G, Uwiera RR. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog. 2015;7:29.
Morgan SJ, Elangbam CS, Berens S, Janovitz E, Vitsky A, Zabka T, et al. Use of animal models of human disease for nonclinical safety assessment of novel pharmaceuticals. Toxicol Pathol. 2013;41:508–18.
Pellegrino D, Bonab AA, Dragotakes SC, Pitman JT, Mariani G, Carter EA. Inflammation and infection: imaging properties of 18F-FDG-labeled white blood cells versus 18F-FDG. J Nucl Med. 2005;46:1522–30.
Bondue B, Sherer F, Van Simaeys G, Doumont G, Egrise D, Yakoub Y, et al. PET/CT with 18F-FDG- and 18F-FBEM-labeled leukocytes for metabolic activity and leukocyte recruitment monitoring in a mouse model of pulmonary fibrosis. J Nucl Med. 2015;56:127–32.
Grabner A, Kentrup D, Edemir B, Sirin Y, Pavenstädt H, Schlatter E, et al. PET with 18F-FDG-labeled T lymphocytes for diagnosis of acute rat renal allograft rejection. J Nucl Med. 2013;54:1147–53.
Tsopelas C. The radiopharmaceutical chemistry of 99mTc-tin fluoride colloid-labeled-leukocytes. Q J Nucl Med Mol Imaging. 2005;49:319–24.
Prinyakupt J, Pluempitiwiriyawej C. Segmentation of white blood cells and comparison of cell morphology by linear and naïve Bayes classifiers. Biomed Eng Online. 2015;14:63.
Swamydas M, Luo Y, Dorf ME, Lionakis MS. Isolation of mouse neutrophils. Curr Protoc Immunol. 2015;110:1–15.
Freitas M, Porto G, Lima JL, Fernandes E. Isolation and activation of human neutrophils in vitro. The importance of the anticoagulant used during blood collection. Clin Biochem. 2008;41:570–5.
Cummings JH, Antoine JM, Azpiroz F, Bourdet-Sicard R, Brandtzaeg P, Calder PC, et al. PASSCLAIM--gut health and immunity. Eur J Nutr. 2004;43(Suppl 2):II118–73.
Alberts B, Johnson A, Lewis J, et al. Molecular biology of the cell. 4th ed. New York: Garland Science; 2002.
Alemán CL, Más RM, Rodeiro I, Noa M, Hernández C, Menéndez R, et al. Reference database of the main physiological parameters in Sprague-Dawley rats from 6 to 32 months. Lab Anim. 1998;32:457–66.
Kim EM, Jeong HJ, Lim ST, Sohn MY. Analysis of Cell fraction of 99mTc-HMPAO radiolabeled leukocytes. Curr Radiopharma. 2020; in press.
Moralidis E, Papakonstantinou E, Arsos G, Boussios N, Koliouskas D, Karakatsanis C. Tc-99m HMPAO labeled white blood cell imaging in a child with eosinophilic lung disease. Clin Nucl Med. 2008;33:38–40.
Yamada S, Kubota K, Kubota R, Ido T, Tamahashi N. High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med. 1995;36:1301–6.
Hammersley PA, Nkohkwo AT. Studies on white blood cell labelling: (99)Tc(m)-HMPAO preferentially labels granulocytes. Nucl Med Commun. 2001;22:981–6.
Sahani DV, Samir AE. Abdominal imaging: expert radiology series. 1st ed. Philadelphia: Saunders; 2010.
Roca M, de Vries EF, Jamar F, Israel O, Signore A. Guidelines for the labelling of leucocytes with (111)In-oxine. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur J Nucl Med Mol Imaging. 2010;37:835–41.
Vargaftig BB, Lefort J, Giroux EL. Haemorrhagic and inflammatory properties of collagenase from C. histolyticum. Agents Actions. 1976;6(5):627–35.
Pires D, Xavier M, Araújo T, Silva JA Jr, Aimbire F, Albertini R. Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci. 2011;26:85–94.
Souza Pinto JC, Remacle-Volon G, Sampaio CA, Damas J. Collagenase-induced oedema in the rat paw and the kinin system. Eur J Pharmacol. 1995;274:101–7.
Marsolais D, Côté CH, Frenette J. Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res. 2001;19:1203–9.
Ertay T, SencanEren M, Karaman M, Oktay G, Durak H. 18F-FDG-PET/CT in initiation and progression of inflammation and infection. Mol Imaging Radionucl Ther. 2017;26:47–52.
Kumar R, Basu S, Torigian D, Anand V, Zhuang H, Alavi A. Role of modern imaging techniques for diagnosis of infection in the era of 18F-fluorodeoxyglucose positron emission tomography. Clin Microbiol Rev. 2008;21:209–24.
Funding
This paper was supported by a Fund of Biomedical Research Institute, Jeonbuk National University Hospital (2019) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI18C0139).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Eun-Mi Kim, Fatima Boud, Phil-Sun Oh, Hwan-Jeong Jeong, Seok Tae Lim and Myung-Hee Shon certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institutional research committee. This article does not contain any studies with human participants performed by any of the authors
Informed Consent
The requirement to obtain informed consent was waived in this non-human-subject study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, EM., Oh, PS., Boud, F. et al. Rodent Leukocyte Isolation and Radiolabeling for Inflammation Imaging Study. Nucl Med Mol Imaging 54, 147–155 (2020). https://doi.org/10.1007/s13139-020-00645-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-020-00645-8