Abstract
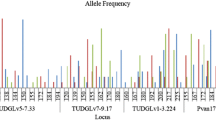
Pacific whiteleg shrimp (Litopenaeus vannamei) is an economically relevant shrimp species in many Asian countries. The specific objective of the current research was to assess microsatellite markers in screening the fast-growth of domesticated L. vannamei stocks to establish a founder population for breeding-selection plans. The postlarvae produced by the reproduction of second generation broodstock were cultured in the same conditions throughout a five months growing period. Ninety juvenile shrimp were selected from the slow-, medium- and the fast-growth groups, and ten microsatellite markers were used to investigate their genetic diversity, and to understand the improvement of a breeding-selection scheme. Ten polymorphic loci (markers) (M1-M10) were produced at ten loci in this sample, among them Primer M8 was the highest polymorphic locus and M7 was the lowest one. A specific locus was found in the fast-growth group using Primer M5. The longest genetic distance (0.481) was determined between the fast- and medium-growth groups and the shortest (0.098) was between the slow- and medium-growth groups; therefore, the largest genetic identity (0.946) was observed between the slow- and medium-growth groups and the smallest (0.667) was observed between the medium- and fast-growth groups. The Unweighted Paired Group with Arithmetic Average (UPGMA) dendrogram based on Nei’s genetic distances provided two different groups; the first consist of the slow- and medium-growth groups and the second the fast-growth group. Selection response and realized heritability for growth were 11.55% and 31.26%, respectively. Therefore, this set of microsatellite markers would provide a useful tool in shrimp breeding schemes.
Similar content being viewed by others
References
Andriantahina F, Liu Xiaolin, Feng Tingting, et al. 2013a. Current status of genetics and genomics of reared penaeid shrimp: information relevant to access and benefit sharing. Marine Biotechnology, 15(4): 399–412
Andriantahina F, Liu Xiaolin, Huang Hao, et al. 2012. Comparison of reproductive performance and offspring quality of domesticated Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 324–325: 194–200
Andriantahina F, Liu Xiaolin, Huang Hao, et al. 2013b. Selection for growth performance of tank-reared Pacific white shrimp, Litopenaeus vannamei. Chinese Journal of Oceanology Limnology, 31(3): 534–541
Arce S M, Moss S M, Argue B J. 2000. Artificial insemination and spawning of Pacific white shrimp, Litopenaeus vannamei: implications for a selective breeding program. In: Tamaru C C T, Tamaru C S, McVey J P, et al., eds. Spawning and Maturation of Aquatic Species: Proceedings of the Twenty-Eighth US-Japan Natural Resources Aquaculture Panel. Hawaii: University of Hawaii Sea Grant College Program. UJNR Technical Report, 28: 5–8
Argue J B, Arce S M, Lotz M J, et al. 2002. Selective breeding of pacific white shrimp (Litopenaeus vannamei) for growth and resistance to Taura syndrome virus. Aquaculture, 204(3–4): 447–460
Benzie J A H. 2009. Use and exchange of genetic resources of penaeid shrimps for food and aquaculture. Reviews in Aquaculture, 1(3–4): 232–250
Benzie J A H, Kenway M, Trott L. 1997. Estimates for the heritability of size in juvenile Penaeus monodon prawns from half-sib matings. Aquaculture, 152(1–4): 49–53
Bhassu S, See L M, Hassan R, et al. 2008. Isolation and characterization of microsatellite loci in the Malaysian giant freshwater prawn, Macrobrachium rosenbergii. Molecular Ecology Resources, 8(5): 983–985
Bierne N, Beuzart I, Vonau V, et al. 2000. Microsatellite-associated heterosis in hatchery-propagated stocks of the shrimp Penaeus stylirostris. Aquaculture, 184(3–4): 203–219
Bruni I. 2010. Il genere Rhododendron L.: diversità genetica e fenomeni di ibridazione (in Italian) [dissertation]. Bologna: Alma Mater Studiorum-Università di Bologna, 130
Chen Limei, Li Qi, Yang Jianmin. 2008. Microsatellite genetic variation in wild and hatchery populations of the sea cucumber (Apostichopus japonicus Selenka) from northern China. Aquaculture Research, 39(14): 1541–1549
Cock J, Gitterle T, Salazar M, et al. 2009. Breeding for disease resistance of Penaeid shrimps. Aquaculture, 286(1–2): 1–11
Coman G J, Arnold S J, Wood A T, et al. 2010. Age: age genetic correlations for weight of Penaeus monodon reared in broodstock tank systems. Aquaculture, 307(1–2): 1–5
Cruz P, Ibarra A M, Mejia-Ruiz H, et al. 2004. Genetic variability assessed by microsatellite in a breeding program of Pacific White Shrimp (Litopenaeus vannamei). Marine Biotechnology, 6(2): 157–164
De Donato M, Manrique R, Ramirez R, et al. 2005. Mass selection and inbreeding effect on a cultivated strain of Penaeus (Litopenaeus) vannamei in Venezuela. Aquaculture, 247(1–4): 159–167
De la Rosa-Vélez J, Escobar R, Correa F, et al. 1999. High allozyme variation and genetic similarity of two populations of commercial penaeids, Penaeus brevirostris (Kingsley) and P. vannamei (Boone), from the Gulf of California. Aquaculture Research, 30(6): 459–463
Divu D, Karunasagar I, Karunasagar I. 2008. Microsatellite DNA markers in the giant freshwater prawn, Macrobrachium rosenbergii: a tool for genetic analysis. Molecular Ecology Resources, 8(5): 1040–1042
Dong Shirui, Kong Jie, Zhang Tianshi, et al. 2006. Parentage determination of Chinese shrimp (Fenneropenaeus chinensis) based on microsatellite DNA markers. Aquaculture, 258(1–4): 283–288
Falconer D S, Macka T F C. 1996. Introduction to Quantitative Genetics. New York: Longman, 438
Farafidy A, Liu Xiaolin, Huang Hao, et al. 2012. Response to selection, heritability and genetic correlations between body weight and body size in Pacific white shrimp, Litopenaeus vannamei. Chinese Journal of Oceanology and Limnology, 30(2): 200–205
Fofana I J, Ofori D, Poitel M, et al. 2009. Diversity and genetic structure of teak (Tectona grandis L.f) in its natural range using DNA microsatellite markers. New Forests, 37(2): 175–195
Garcia D K, Benzie J A H. 1995. RAPD markers of potential use in penaeid prawn (Penaeus monodon) breeding programs. Aquaculture, 130(2–3): 137–144
Gitterle T, Rye M, Salte R, et al. 2005. Genetic (co)variation in harvest body weight and survival in Penaeus (Litopenaeus) vannamei under standard commercial conditions. Aquaculture, 243(1–4): 83–92
Gjedrem T, Fimland E. 1995. Potential benefits from high health and genetically improved shrimp stocks. In: Browdy C L, Hopkins J S, eds. Swimming Through Water. Proceedings of Special Session on Shrimp Farming, 95: 235
Goyard E, Goarant C, Ansquer D, et al. 2008. Cross breeding of different domesticated lines as a simple way for genetic improvement in small aquaculture industries: Heterosis and inbreeding effects on growth and survival rates of the Pacific blue shrimp Penaeus (Litopenaeus) stylirostris. Aquaculture, 278(1–4): 43–50
Goyard E, Patrois J, Peignon J M, et al. 2002. Selection for better growth of Penaeus stylirostris in Tahiti and New Caledonia. Aquaculture, 204(3–4): 461–468
Hetzel D J S, Crocos P J, Davis G P, et al. 2000. Response to selection and heritability for growth in the Kuruma prawn, Penaeus japonicus. Aquaculture, 181(3–4): 215–223
Hoa N D. 2009. Domestication of black tiger shrimp (Penaeus monodon) in recirculation systems in Vietnam [dissertation]. Ghent, Belgium: Ghent University, 179
Jerry D R, Preston N P, Crocos P J, et al. 2004. Parentage determination of Kuruma shrimp Penaeus (Marsupenaeus) japonicus using microsatellite markers (Bate). Aquaculture, 235(1–4): 237–247
Jung H, Lyons R E, Dinh H, et al. 2011. Transcriptomics of a giant freshwater prawn (Macrobrachium rosenbergii): de novo assembly, annotation and marker discovery. PLoS ONE, 6(12): e27938
Karaket T, Poompuang S, Na-Nakorn U, et al. 2011. DNA microsatellite-based evaluation of early growth performance among strains of freshwater prawn Macrobrachium rosenbergii de Man. Aquaculture, 311(1–4): 115–122
Kelly M W, Rhymer J M. 2005. Population genetic structure of a rare unionid (Lampsilis cariosa) in a recently glaciated landscape. Conservation Genetics, 6(5): 789–802
Keys S J, Crocos P J, Burridge C Y, et al. 2004. Comparative growth and survival of inbred and outbred Penaeus (marsupenaeus) japonicus, reared under controlled environment conditions: indications of inbreeding depression. Aquaculture, 241(1–4): 151–168
Khan S R, Akter H, Sultana N, et al. 2014. Genetic diversity in three river populations of the giant freshwater prawn (Macrobrachium rosenbergii) in Bangladesh assessed by microsatellite DNA markers. International Journal of Agriculture & Biology, 16(1): 195–200
Kheirabadi K, Alijani S, Zavadilová L, et al. 2013. Estimation of genetic parameters for daily milk yields of primiparous Iranian Holstein cows. Archiv Tierzucht, 56(44): 455–466
Krishna G, Gopikrishna G, Gopal C, et al. 2011. Genetic parameters for growth and survival in Penaeus monodon cultured in India. Aquaculture, 318(1–2): 74–78
Liu Z J, Cordes J F. 2004. DNA marker technologies and their applications in aquaculture genetics. Aquaculture, 238(1–4): 1–37
Liu Feng, Xia Junhong, Bai Zhiyi, et al. 2009. High genetic diversity and substantial population differentiation in grass carp (Ctenopharyngodon idella) revealed by microsatellite analysis. Aquaculture, 297(1–4): 51–56
Ma K Y, Feng J B, Li J L. 2012a. Genetic variation based on microsatellite analysis of the oriental river prawn, Macrobrachium nipponense from Qiandao Lake in China. Genetics and Molecular Research, 11(4): 4235–4244
Ma Keyi, Qiu Gaofeng, Feng Jianbin, et al. 2012b. Transcriptome analysis of the oriental river prawn, Macrobrachium nipponense using 454 pyrosequencing for discovery of genes and markers. PLoS One, 7(6): e39727
Moore S S, Whan V, Davis G P, et al. 1999. The development and application of genetic markers for the Kuruma prawn Penaeus japonicus. Aquaculture, 173(1–4): 19–32
Moss D R, Arce S M, Otoshi C A, et al. 2007. Effects of inbreeding on survival and growth of pacific white shrimp Penaeus (Litopenarus) vannamei. Aquaculture, 272(S1): S30–S37
Nei M. 1972. Genetic distance between populations. The American Naturalist, 106(949): 283–292
Nei M, Chakraborty R, Fuerst P A. 1976. Infinite allele model with varying mutation rate. Proceeding of the National Academy of Sciences of the United States of America, 73(11): 4164–4168
Nelson K, Hedgecock D. 1980. Enzyme polymorphism and adaptive strategy in the decapod Crustacea. The American Naturalist, 116(2): 238–280
Pourkazemi M, Nazari S, Bakhshalizadeh S. 2010. Karyotype analysis in white bream (Blicca bjoerkna transcaucasica) from north coast of Iran. Iranian Journal of Fisheries Sciences, 9(3): 454–463
Rezvani Gilkolaei S, Safari R, Laloei F, et al. 2011. Using RAPD markers potential to identify heritability for growth in Fenneropenaeus indicus. Iranian Journal of Fisheries Sciences, 10(1): 123–134
Rousset F. 2008. Genepop’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Molecular Ecology Resources, 8(1): 103–106
Sambrook J, Russell D W. 2001. Molecular Cloning: A Laboratory Manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press, 132–144
Schneider K J, Tidwell J H, Gomelsky B, et al. 2012. Genetic diversity of cultured and wild populations of the giant freshwater prawn Macrobrachium rosenbergii (de Man, 1879) based on microsatellite analysis. Aquaculture Research, 44(9): 1425–1437
Sriphairoj K, Kamonrat W, Na-korn U. 2007. Genetic aspect in broodstock management of the critically endangered Makong giant catfish, Pangasianodon gigas in Thailand. Aquaculture, 264(1–4): 34–46
Szeker K. 2012. Nucleoside phosphorylases from thermophiles-Recombinant expression and biocatalytic use for modified nucleosides [dissertation]. Berlin: Technischen Universität, 152
Tamura K, Dudley J, Nei M, et al. 2007. MEGA4: Molecular and Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24(8): 1596–1599
Taris N, Batista F M, Boudry P. 2007. Evidence of response to unintentional selection for faster development and inbreeding depression in Crassostrea gigas larvae. Aquaculture, 272(S1): S69–S79
Van Oosterhout C, Hutchinson W F, Wills D P M, et al. 2004. Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes, 4(3): 535–538
Vaseeharan B, Rajakamaran P, Jayaseelan D, et al. 2013. Molecular markers and their application in genetic diversity of penaeid shrimp. Aquaculture International, 21(2): 219–241
Wang Le, Meng Zining, Liu Xiaochun, et al. 2011. Genetic diversity and differentiation of the orange-spotted grouper (Epinephelus coioides) between and within cultured stocks and wild populations inferred from microsatellite DNA analysis. International Journal of Molecular Sciences, 12(7): 4378–4394
Wolfus G M, Garcia D K, Alcivar-Warren A. 1997. Application of the microsatellite technique for analyzing genetic diversity in shrimp breeding programs. Aquaculture, 152(1–4): 35–47
Wuthisuthimethavee S, Lumubol P, Vanavichit A, et al. 2003. Development of microsatellite markers in black tiger shrimp (Penaeus monodon Fabricius). Aquaculture, 224(1–4): 39–50
Xu Zhenkang, Primavera J H, de La Pena L D, et al. 2001. Genetic diversity of wild and cultured black tiger shrimp (Penaeus monodon) in the Philippines using microsatellites. Aquaculture, 199(1–2): 13–40
You E-M, Chiu T-S, Liu K-F, et al. 2008. Microsatellite and mitochondrial haplotype diversity reveals population differentiation in the tiger shrimp (Penaeus monodon) in the Indo-Pacific region. Animal Genetics, 39(3): 267–277
Yue Genhua, Zhu Zeyuan, Lo L C, et al. 2009. Genetic variation and population structure of Asian seabass (Lates calcarifer) in the Asia-Pacific region. Aquaculture, 293(1–2): 22–28
Zima Jr J, Leština D, Konvička M. 2013. Characterization of ten polymorphic microsatellite markers for an endangered butterfly Argynnis niobe and their cross-species utility in the closely related species A. adippe (Lepidoptera: Nymphalidae). European Journal of Entomology, 110(2): 383–387
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: The Agricultural Science and Technology Achievement Transformation Fund Project of Ministry of Science and Technology, China under contract No. 2012GB2E200361; the Key Laboratory of Marine Biology, Institute of Oceanlogy, Chinese Academy of Sciences; Qianjiang distinguished experts of Hangzhou; Extension and Demonstration Program in Desalting Culture of Litopenaeus Vannamei New Breed “Ke Hai 1”, Northwest A&F University, China under contract No. XNY2013-4.
Rights and permissions
About this article
Cite this article
Andriantahina, F., Liu, X. & Huang, H. Using microsatellite markers to identify heritability of Pacific whiteleg shrimp Litopenaeus vannamei . Acta Oceanol. Sin. 34, 59–65 (2015). https://doi.org/10.1007/s13131-015-0688-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-015-0688-6