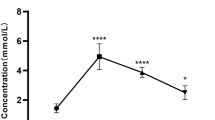
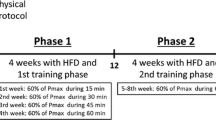
Abstract
Lactate, an important exercise metabolite, induces white adipose tissue browning by upregulated uncoupling protein 1 (UCP1) expression. However, the function of lactate during browning of inguinal white adipose tissue (iWAT) caused by exercise is unclear. Here, we considered lactate as an exercise supplement and investigated the effects of chronic pre-exercise lactate administration on energy metabolism and adipose tissue browning. C57B/L6 male mice (5 weeks of age) were divided into six groups. We evaluated the changes in blood lactate levels in each group of mice after the intervention. Energy expenditure was measured after the intervention immediately by indirect calorimetry. The marker protein levels and gene expressions were determined by western-blot and quantitative real-time PCR. HIIT significantly decreased adipose tissue weight while increased energy expenditure and the expression of UCP1 in iWAT; however, these regulations were inhibited in the DCA+HIIT group. Compared with the MICT and LAC groups, long-term lactate injection before MICT led to lower WAT weight to body weight ratios and higher energy expenditure in mice. Furthermore, the marker genes of browning in iWAT, such as Ucp1 and Pparγ, were significantly increased in the LAC+MICT group than in the other groups, and the expression of monocarboxylate transporter-1 (Mct1) mRNA was also significantly increased. Lactate was involved in exercise-mediated browning of iWAT, and its mechanism might be the increased of lactate transport through MCT1 or PPARγ upregulation induced by exercise. These findings suggest exogenous lactate may be a new exercise supplement to regulate metabolism.






Similar content being viewed by others
Data availability
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.
References
Brooks GA (2020) Lactate as a fulcrum of metabolism. Redox Biol 35:101454. https://doi.org/10.1016/j.redox.2020.101454
Carriere A, Jeanson Y, Berger-Muller S, Andre M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, Villageois P, Louche K, Collas P, Moro C, Dani C, Villarroya F, Casteilla L (2014) Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63:3253–3265. https://doi.org/10.2337/db13-1885
Carriere A, Lagarde D, Jeanson Y, Portais JC, Galinier A, Ader I, Casteilla L (2020) The emerging roles of lactate as a redox substrate and signaling molecule in adipose tissues. J Physiol Biochem 76:241–250. https://doi.org/10.1007/s13105-019-00723-2
Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB (2014) Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med 12:215. https://doi.org/10.1186/s12916-014-0215-1
DiGirolamo M, Newby FD, Lovejoy J (1992) Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J 6:2405–2412. https://doi.org/10.1096/fasebj.6.7.1563593
Golde WT, Gollobin P, Rodriguez LL (2005) A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 34:39–43. https://doi.org/10.1038/laban1005-39
Hashimoto T, Brooks GA (2008) Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med Sci Sports Exerc 40:486–494. https://doi.org/10.1249/MSS.0b013e31815fcb04
Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA (2007) Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21:2602–2612. https://doi.org/10.1096/fj.07-8174com
Hojman P, Brolin C, Norgaard-Christensen N, Dethlefsen C, Lauenborg B, Olsen CK, Abom MM, Krag T, Gehl J, Pedersen BK (2019) IL-6 release from muscles during exercise is stimulated by lactate-dependent protease activity. Am J Physiol Endocrinol Metab 316:E940–E947. https://doi.org/10.1152/ajpendo.00414.2018
Hoshino D, Hanawa T, Takahashi Y, Masuda H, Kato M, Hatta H (2014) Chronic post-exercise lactate administration with endurance training increases glycogen concentration and monocarboxylate transporter 1 protein in mouse white muscle. J Nutr Sci Vitaminol (Tokyo) 60:413–419. https://doi.org/10.3177/jnsv.60.413
Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Zhan L, Yanxiang GJ, White E, Rabinowitz JD (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551:115–118. https://doi.org/10.1038/nature24057
Jansson PA, Larsson A, Smith U, Lonnroth P (1994) Lactate release from the subcutaneous tissue in lean and obese men. J Clin Invest 93:240–246. https://doi.org/10.1172/JCI116951
Khalafi M, Mohebbi H, Symonds ME, Karimi P, Akbari A, Tabari E, Faridnia M, Moghaddami K (2020) The impact of moderate-intensity continuous or high-intensity interval training on adipogenesis and browning of subcutaneous adipose tissue in obese male rats. Nutrients 12. https://doi.org/10.3390/nu12040925
Kitaoka Y, Takeda K, Tamura Y, Hatta H (2016) Lactate administration increases mRNA expression of PGC-1alpha and UCP3 in mouse skeletal muscle. Appl Physiol Nutr Metab 41:695–698. https://doi.org/10.1139/apnm-2016-0016
Lagarde D, Jeanson Y, Barreau C, Moro C, Peyriga L, Cahoreau E, Guissard C, Arnaud E, Galinier A, Bouzier-Sore AK, Pellerin L, Chouchani ET, Penicaud L, Ader I, Portais JC, Casteilla L, Carriere A (2021) Lactate fluxes mediated by the monocarboxylate transporter-1 are key determinants of the metabolic activity of beige adipocytes. J Biol Chem 296:100137. https://doi.org/10.1074/jbc.RA120.016303
Lezi E, Lu J, Selfridge JE, Burns JM, Swerdlow RH (2013) Lactate administration reproduces specific brain and liver exercise-related changes. J Neurochem 127:91–100. https://doi.org/10.1111/jnc.12394
Liang F, Huang T, Li B, Zhao Y, Zhang X, Xu B (2020) High-intensity interval training and moderate-intensity continuous training alleviate beta-amyloid deposition by inhibiting NLRP3 inflammasome activation in APPswe/PS1dE9 mice. Neuroreport 31:425–432. https://doi.org/10.1097/WNR.0000000000001429
Lund J, Aas V, Tingstad RH, Van Hees A, Nikolic N (2018) Utilization of lactic acid in human myotubes and interplay with glucose and fatty acid metabolism. Sci Rep 8:9814. https://doi.org/10.1038/s41598-018-28249-5
Maillard F, Pereira B, Boisseau N (2018) Effect of high-intensity interval training on total, abdominal and visceral fat mass: a meta-analysis. Sports Med 48:269–288. https://doi.org/10.1007/s40279-017-0807-y
Martinez-Tellez B, Sanchez-Delgado G, Acosta FM, Alcantara JMA, Amaro-Gahete FJ, Martinez-Avila WD, Merchan-Ramirez E, Munoz-Hernandez V, Osuna-Prieto FJ, Jurado-Fasoli L, Xu H, Ortiz-Alvarez L, Arias-Tellez MJ, Mendez-Gutierrez A, Labayen I, Ortega FB, Schonke M, Rensen PCN, Aguilera CM et al (2022) No evidence of brown adipose tissue activation after 24 weeks of supervised exercise training in young sedentary adults in the ACTIBATE randomized controlled trial. Nat Commun 13:5259. https://doi.org/10.1038/s41467-022-32502-x
Motta VF, Bargut TL, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA (2019) Browning is activated in the subcutaneous white adipose tissue of mice metabolically challenged with a high-fructose diet submitted to high-intensity interval training. J Nutr Biochem 70:164–173. https://doi.org/10.1016/j.jnutbio.2019.05.008
Mu WJ, Zhu JY, Chen M, Guo L (2021) Exercise-mediated browning of white adipose tissue: its significance, mechanism and effectiveness. Int J Mol Sci 22. https://doi.org/10.3390/ijms222111512
Nikooie R, Samaneh S (2016) Exercise-induced lactate accumulation regulates intramuscular triglyceride metabolism via transforming growth factor-beta1 mediated pathways. Mol Cell Endocrinol 419:244–251. https://doi.org/10.1016/j.mce.2015.10.024
Ohno Y, Ando K, Ito T, Suda Y, Matsui Y, Oyama A, Kaneko H, Yokoyama S, Egawa T, Goto K (2019) Lactate stimulates a potential for hypertrophy and regeneration of mouse skeletal muscle. Nutrients 11. https://doi.org/10.3390/nu11040869
Oishi Y, Tsukamoto H, Yokokawa T, Hirotsu K, Shimazu M, Uchida K, Tomi H, Higashida K, Iwanaka N, Hashimoto T (1985) (2015) Mixed lactate and caffeine compound increases satellite cell activity and anabolic signals for muscle hypertrophy. J Appl Physiol 118:742–749. https://doi.org/10.1152/japplphysiol.00054.2014
Pedersen MGB, Sondergaard E, Nielsen CB, Johannsen M, Gormsen LC, Moller N, Jessen N, Rittig N (2022) Oral lactate slows gastric emptying and suppresses appetite in young males. Clin Nutr 41:517–525. https://doi.org/10.1016/j.clnu.2021.12.032
Rundqvist H, Velica P, Barbieri L, Gameiro PA, Bargiela D, Gojkovic M, Mijwel S, Reitzner SM, Wulliman D, Ahlstedt E, Ule J, Ostman A, Johnson RS (2020) Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. Elife 9. https://doi.org/10.7554/eLife.59996
Ruschke K, Fishbein L, Dietrich A, Kloting N, Tonjes A, Oberbach A, Fasshauer M, Jenkner J, Schon MR, Stumvoll M, Bluher M, Mantzoros CS (2010) Gene expression of PPARgamma and PGC-1alpha in human omental and subcutaneous adipose tissues is related to insulin resistance markers and mediates beneficial effects of physical training. Eur J Endocrinol 162:515–523. https://doi.org/10.1530/EJE-09-0767
Severinsen MCK, Pedersen BK (2020) Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev 41:594–609. https://doi.org/10.1210/endrev/bnaa016
Sonestedt E, Wirfalt E, Wallstrom P, Gullberg B, Orho-Melander M, Hedblad B (2011) Dairy products and its association with incidence of cardiovascular disease: the Malmo diet and cancer cohort. Eur J Epidemiol 26:609–618. https://doi.org/10.1007/s10654-011-9589-y
Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Nigro P, Ryan RE, Xue R, Sakaguchi M, Lynes MD, So K, Mul JD, Lee MY, Balan E, Pan H, Dreyfuss JM, Hirshman MF, Azhar M, Hannukainen JC, Nuutila P et al (2019) TGF-beta2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab 1:291–303. https://doi.org/10.1038/s42255-018-0030-7
Thomas C, Bishop D, Moore-Morris T, Mercier J (2007) Effects of high-intensity training on MCT1, MCT4, and NBC expressions in rat skeletal muscles: influence of chronic metabolic alkalosis. Am J Physiol Endocrinol Metab 293:E916–E922. https://doi.org/10.1152/ajpendo.00164.2007
Wang N, Liu Y, Ma Y, Wen D (2017) High-intensity interval versus moderate-intensity continuous training: superior metabolic benefits in diet-induced obesity mice. Life Sci 191:122–131. https://doi.org/10.1016/j.lfs.2017.08.023
Yao Z, Yan Y, Zheng X, Wang M, Zhang H, Li H, Chen W (2020) Dietary lactate supplementation protects against obesity by promoting adipose browning in mice. J Agric Food Chem 68:14841–14849. https://doi.org/10.1021/acs.jafc.0c05899
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 31871207).
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used. YL and XC performed the experiments, ran the statistical analyses, and drafted the manuscript; JZ contributed to perform the experiments and write the manuscript; WH and JS participated in performing of the experiments; JZ participated in design of the experiments and draft of manuscript. All the authors have read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare there are no competing interests.
Ethics approval
All animal care and experimental protocols complied with the guidelines of the Ethics and Animal Welfare Committee of Beijing Normal University (Approval No. CLS-EAW-2015-002).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Lactate produced by HIIT could induce the browning of iWAT in mice.
• MICT supplemented with lactate could promote iWAT browning and increase the energy metabolism.
• The promotive effect of lactate on browning in exercise was related to the activation of the PPARγ and MCT1 pathways.
Supplementary information
ESM 1
(DOCX 14 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Li, Y., Zhang, J. et al. Lactate coordinated with exercise promoted the browning of inguinal white adipose tissue. J Physiol Biochem 80, 303–315 (2024). https://doi.org/10.1007/s13105-023-01004-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-01004-9