Abstract
We have previously shown in renal cells that expression of the water channel Aquaporin-2 increases cell proliferation by a regulatory volume mechanism involving Na+/H+ exchanger isoform 2. Here, we investigated if Aquaporin-2 (AQP2) also modulates Na+/H+ exchanger isoform 1-dependent cell proliferation. We use two AQP2-expressing cortical collecting duct models: one constitutive (WT or AQP2-transfected RCCD1 cell line) and one inducible (control or vasopressin-induced mpkCCDc14 cell line). We found that Aquaporin-2 modifies Na+/H+ exchanger isoform 1 (NHE1) contribution to cell proliferation. In Aquaporin-2-expressing cells, Na+/H+ exchanger isoform 1 is anti-proliferative at physiological pH. In acid media, Na+/H+ exchanger isoform 1 contribution turned from anti-proliferative to proliferative only in AQP2-expressing cells. We also found that, in AQP2-expressing cells, NHE1-dependent proliferation changes parallel changes in stress fiber levels: at pH 7.4, Na+/H+ exchanger isoform 1 would favor stress fiber disassembly and, under acidosis, NHE1 would favor stress fiber assembly. Moreover, we found that Na+/H+ exchanger-dependent effects on proliferation linked to Aquaporin-2 relied on Transient Receptor Potential Subfamily V calcium channel activity. In conclusion, our data show that, in collecting duct cells, the water channel Aquaporin-2 modulates NHE1-dependent cell proliferation. In AQP2-expressing cells, at physiological pH, the Na+/H+ exchanger isoform 1 function is anti-proliferative and, at acidic pH, Na+/H+ exchanger isoform 1 function is proliferative. We propose that Na+/H+ exchanger isoform 1 modulates proliferation through an interplay with stress fiber formation.








Similar content being viewed by others
References
Beaty BT, Wang Y, Bravo-Cordero JJ, Sharma VP, Miskolci V, Hodgson L, Condeelis J (2014) Talin regulates moesin-NHE-1 recruitment to invadopodia and promotes mammary tumor metastasis. J Cell Biol 205:737–751. https://doi.org/10.1083/jcb.201312046
Boudaoud A, Burian A, Borowska-Wykret D, Uyttewaal M, Wrzalik R, Kwiatkowska D, Hamant O (2014) FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat Protoc 9:457–463. https://doi.org/10.1038/nprot.2014.024
Counillon L, Pouyssegur J (2000) The expanding family of eucaryotic Na(+)/H(+) exchangers. J Biol Chem 275:1–4
Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL (2000) Direct binding of the Na--H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol Cell 6:1425–1436
Di Giusto G, Flamenco P, Rivarola V, Fernandez J, Melamud L, Ford P, Capurro C (2012) Aquaporin 2-increased renal cell proliferation is associated with cell volume regulation. J Cell Biochem 113:3721–3729. https://doi.org/10.1002/jcb.24246
Flamenco P, Galizia L, Rivarola V, Fernandez J, Ford P, Capurro C (2009) Role of AQP2 during apoptosis in cortical collecting duct cells. Biol Cell 101:237–250. https://doi.org/10.1042/BC20080050
Ford P, Rivarola V, Chara O, Blot-Chabaud M, Cluzeaud F, Farman N, Parisi M, Capurro C (2005) Volume regulation in cortical collecting duct cells: role of AQP2. Biol Cell 97:687–697. https://doi.org/10.1042/BC20040116
Ford P, Rivarola V, Kierbel A, Chara O, Blot-Chabaud M, Farman N, Parisi M, Capurro C (2002) Differential role of Na+/H+ exchange isoforms NHE-1 and NHE-2 in a rat cortical collecting duct cell line. J Membr Biol 190:117–125. https://doi.org/10.1007/s00232-002-1030-8
Galan-Cobo A, Ramirez-Lorca R, Toledo-Aral JJ, Echevarria M (2016) Aquaporin-1 plays important role in proliferation by affecting cell cycle progression. J Cell Physiol 231:243–256. https://doi.org/10.1002/jcp.25078
Grinstein S, Smith JD, Onizuka R, Cheung RK, Gelfand EW, Benedict S (1988) Activation of Na+/H+ exchange and the expression of cellular proto-oncogenes in mitogen- and phorbol ester-treated lymphocytes. J Biol Chem 263:8658–8665
Jenkins EC Jr, Debnath S, Gundry S, Uyar U, Fata JE (2012) Intracellular pH regulation by Na(+)/H(+) exchanger-1 (NHE1) is required for growth factor-induced mammary branching morphogenesis. Dev Biol 365:71–81. https://doi.org/10.1016/j.ydbio.2012.02.010
Jensen HH, Pedersen GA, Morgen JJ, Parsons M, Pedersen SF, Nejsum LN (2019) The Na(+) /H(+) exchanger NHE1 localizes as clusters to cryptic lamellipodia and accelerates collective epithelial cell migration. J Physiol 597:849–867. https://doi.org/10.1113/JP277383
Lagana A, Vadnais J, Le PU, Nguyen TN, Laprade R, Nabi IR, Noel J (2000) Regulation of the formation of tumor cell pseudopodia by the Na(+)/H(+) exchanger NHE1. J Cell Sci 113(Pt 20):3649–3662
Lang F, Foller M, Lang K, Lang P, Ritter M, Vereninov A, Szabo I, Huber SM, Gulbins E (2007) Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol 428:209–225. https://doi.org/10.1016/S0076-6879(07)28011-5
Lee WH, Choong LY, Jin TH, Mon NN, Chong S, Liew CS, Putti T, Lu SY, Harteneck C, Lim YP (2017) TRPV4 plays a role in breast cancer cell migration via Ca(2+)-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis 6:e338. https://doi.org/10.1038/oncsis.2017.39
Li K, Su W, Li M, Chen CJ, Li YY, Lai LY, Zhang MM, Liu SJ, Fichna J, Peng A, Hao CM, Gu Y, Lin SY (2013) Acid loading stimulates rat glomerular mesangial cells proliferation through Na(+)-H (+) exchanger isoform 1 (NHE1)-dependent pathway. Naunyn Schmiedebergs Arch Pharmacol 386:563–569. https://doi.org/10.1007/s00210-013-0856-1
Li W, Jin WW, Tsuji K, Chen Y, Nomura N, Su L, Yui N, Arthur J, Cotecchia S, Paunescu TG, Brown D, Lu HAJ (2017) Ezrin directly interacts with AQP2 and promotes its endocytosis. J Cell Sci 130:2914–2925. https://doi.org/10.1242/jcs.204842
Pizzoni A, Lopez Gonzalez M, Di Giusto G, Rivarola V, Capurro C, Ford P (2018) AQP2 can modulate the pattern of Ca(2+) transients induced by store-operated Ca(2+) entry under TRPV4 activation. J Cell Biochem 119:4120–4133. https://doi.org/10.1002/jcb.26612
Putney LK, Barber DL (2003) Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem 278:44645–44649. https://doi.org/10.1074/jbc.M308099200M308099200
Putney LK, Denker SP, Barber DL (2002) The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol 42:527–552. https://doi.org/10.1146/annurev.pharmtox.42.092001.14380142/1/527
Rivarola V, Di Giusto G, Christensen MJ, Ford P, Capurro C (2017) AQP2-induced acceleration of renal cell proliferation involves the activation of a regulatory volume increase mechanism dependent on NHE2. J Cell Biochem 118:967–978. https://doi.org/10.1002/jcb.25602
Rivarola V, Flamenco P, Melamud L, Galizia L, Ford P, Capurro C (2010) Adaptation to alkalosis induces cell cycle delay and apoptosis in cortical collecting duct cells: role of Aquaporin-2. J Cell Physiol 224:405–413. https://doi.org/10.1002/jcp.22136
Roos A, Boron WF (1981) Intracellular pH. Physiol Rev 61:296–434
Sanhueza C, Araos J, Naranjo L, Toledo F, Beltran AR, Ramirez MA, Gutierrez J, Pardo F, Leiva A, Sobrevia L (2017) Sodium/proton exchanger isoform 1 regulates intracellular pH and cell proliferation in human ovarian cancer. Biochim Biophys Acta 1863:81–91. https://doi.org/10.1016/j.bbadis.2016.10.013
Schelling JR, Abu Jawdeh BG (2008) Regulation of cell survival by Na+/H+ exchanger-1. Am J Physiol Renal Physiol 295:F625–F632. https://doi.org/10.1152/ajprenal.90212.2008
Stock C, Pedersen SF (2017) Roles of pH and the Na(+)/H(+) exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective? Semin Cancer Biol 43:5–16. https://doi.org/10.1016/j.semcancer.2016.12.001
Sutka M, Amodeo G, Ozu M (2017) Plant and animal aquaporins crosstalk: what can be revealed from distinct perspectives. Biophys Rev 9:545–562. https://doi.org/10.1007/s12551-017-0313-3
Suzuki M, Mizuno A, Kodaira K, Imai M (2003) Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278:22664–22668. https://doi.org/10.1074/jbc.M302561200
Tavares S, Vieira AF, Taubenberger AV, Araujo M, Martins NP, Bras-Pereira C, Polonia A, Herbig M, Barreto C, Otto O, Cardoso J, Pereira-Leal JB, Guck J, Paredes J, Janody F (2017) Actin stress fiber organization promotes cell stiffening and proliferation of pre-invasive breast cancer cells. Nat Commun 8:15237. https://doi.org/10.1038/ncomms15237
Valles PG, Bocanegra V, Gil Lorenzo A, Costantino VV (2015) Physiological functions and regulation of the Na+/H+ exchanger [NHE1] in renal tubule epithelial cells. Kidney Blood Press Res 40:452–466. https://doi.org/10.1159/000368521
Vexler ZS, Symons M, Barber DL (1996) Activation of Na+-H+ exchange is necessary for RhoA-induced stress fiber formation. J Biol Chem 271:22281–22284. https://doi.org/10.1074/jbc.271.37.22281
Vrenken KS, Jalink K, van Leeuwen FN, Middelbeek J (2016) Beyond ion-conduction: Channel-dependent and -independent roles of TRP channels during development and tissue homeostasis. Biochim Biophys Acta 1863:1436–1446. https://doi.org/10.1016/j.bbamcr.2015.11.008
Wu KL, Khan S, Lakhe-Reddy S, Wang L, Jarad G, Miller RT, Konieczkowski M, Brown AM, Sedor JR, Schelling JR (2003) Renal tubular epithelial cell apoptosis is associated with caspase cleavage of the NHE1 Na+/H+ exchanger. Am J Physiol Renal Physiol 284:F829–F839. https://doi.org/10.1152/ajprenal.00314.200200314.2002
Yu L, Hales CA (2011) Silencing of sodium-hydrogen exchanger 1 attenuates the proliferation, hypertrophy, and migration of pulmonary artery smooth muscle cells via E2F1. Am J Respir Cell Mol Biol 45:923–930. https://doi.org/10.1165/rcmb.2011-0032OC
Acknowledgments
The authors thank Ricardo Dorr for his technical assistance.
Funding
This study was funded by grants from Fondo Nacional para la Ciencia y la Tecnología, Argentina [Grant Number: PICT 15-3525]; Universidad de Buenos Aires, Argentina [Grant Number: UBACYT 20020170100451BA and UBACYT 20020130100697BA]; and Consejo Nacional de Ciencia y Tecnología, Argentina [Grant Number: PIP 112 20130100057CO].
Author information
Authors and Affiliations
Contributions
Marina Mazzocchi and Gisela Di Giusto should be considered joint first authors: They have performed the majority of the experimental work. Micaela Porta performed some of the proliferation experiments. Alejandro Pizzoni has provided experimental design ideas and has worked in the microscope setup. Natalia Beltramone has participated in setting up the mpkCCDc14 cell model in nonpermeable supports. Paula Ford and Claudia Capurro have helped in the interpretation of the data and in revising the manuscript critically for important intellectual content. Valeria Rivarola has directed all the work providing the de majority of the design of the ideas and the experiments, the interpretation of the data, preparation of the manuscript and revising it critically for important intellectual content
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marina Mazzocchi and Gisela Di Giusto should be considered joint first authors.
Key points
• NHE1 activity in AQP2-expressing cells is anti-proliferative at pH = 7.4 and proliferative at pH = 7.0.
• In the presence of AQP2, NHE1-dependent stress fiber formation modulates proliferation.
• Interaction between AQP2-TRPV calcium channels modulates NHE1-dependent proliferation.
Electronic supplementary material
Supplementary Fig. 1
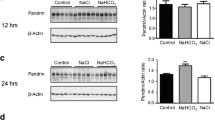
AQP2 protein expression in mpkCDc14cells. Cells were exposed to vehicle (control) or 1 nM AVP (+AVP) for four days before experiments were performed. a: Representative images obtained after immunofluorescence studies using specific antibodies target to AQP2. b: Densitometric quantification of total membrane immunoblot bands, expressed as the AQP2 / β-Actin ratio. Bars are mean ± SEM from three independent experiments. ** p < 0.01, n = 3 when comparing control vs. AVP-stimulated mpkCCDc14 cells. Inset: Representative immunoblot using anti-AQP2 (28kD band) or β-Actin (42kD band) antibodies (PNG 621 kb)
Rights and permissions
About this article
Cite this article
Mazzocchi, M., Di Giusto, G., Porta, M. et al. Na+/H+ exchanger isoform 1 activity in AQP2-expressing cells can be either proliferative or anti-proliferative depending on extracellular pH. J Physiol Biochem 76, 37–48 (2020). https://doi.org/10.1007/s13105-019-00713-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-019-00713-4