Abstract
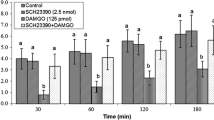
This study was designed to examine the effects of intracerebroventricular injection of DL-AP5 (N-methyl-D-aspartate (NMDA) receptor antagonist) and glutamate on ghrelin-induced feeding behavior in 3-h food-deprived (FD3) broiler cockerels. At first, guide cannula was surgically implanted in the right lateral ventricle of chickens. In experiment 1, birds were intracerebroventricularly injected with 0, 2.5, 5, and 10 nmol of DL-AP5. In experiment 2, chickens received 5 nmol DL-AP5 prior to the injection of 0.6 nmol ghrelin. In experiment 3, birds were administered with 0.6 nmol ghrelin after 300 nmol glutamate, and the cumulative feed intake was determined at 3-h postinjection. The results of this study showed that the intracerebroventricular injection of DL-AP5 increased food consumption in FD3 broiler cockerels (P ≤ 0.05), and this increase occurs in a dose-dependent manner. Moreover, the decreased food intake induced with the intracerebroventricular injection of ghrelin was additively enhanced by pretreatment with glutamate, and this effect was attenuated by DL-AP5 administration(P ≤ 0.05).These results suggest that there is an interaction between ghrelin and glutamatergic system (through NMDA receptor) on food intake in broiler cockerels.



Similar content being viewed by others
References
Broadwell RD, Brightman MW (1976) Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol 166(3):257–283
Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ (2001) The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord 25(5):63–67
Date Y, Murakami N, Toshinai K, Matsukara S, Niijima A, Matsuda Kangawa K, Nakazato M (2002) The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123:1120–1128
Denbow DM, Cherry JA, Siegel PB, Van Kery HP (1981) Eating, drinking and temperature response of chicks to brain catecholamine injections. Physiol Behav 27:265–269
Hagan MM, Rushing PA, Schwartz MW, Yagaloff KA, Burn P, Woods SC, Seeley RJ (1999) Role of the CNS melanocortin system in the response to overfeeding. J Neurosci 19:2362–2367
Kaiya H, Vander Geyten S, Kojima M, Hosoda H, Kitajima Y, Matsumoto M, Geelissen S, Darras VM, Kangawa K (2002) Ghrelin: purification cDNA cloning and biological activity. Endocrinol 143:3454–3463
Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS (1999) Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20:68–100
Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I (2001) Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and agouti-related mRNA levels and body weight in rats. Diabetes 50:2438–2443
Khan MSI, Dodo K, Yahata K, Nishimoto S, Ueda H, Taneike T, Kitazawa T, Hosaka Y, Bungo T (2006) Intracerebroventricular administration of growth hormone releasing peptide-6 ( GHRP-6) inhibits food intake, but not food retention of crop and stomach in neonatal chicks. J Poult Sci 43:35–40
Kiss J, Csaba Z, Csaki A, Halasz B (2005) Glutamatergic innervation of neuropeptide Y and pro-opiomelanocortin-containing neurons in the hypothalamic arcuate nucleus of the rat. Eur J Neurosci 21:2111–2119
Kojima M, Kangawa K (2005) Ghrelin: structure and function. Physiol Rev 85:495–522
Marks DL, Cone RD (2001) Central melanocortins and the regulation of weight during acute and chronic disease. Recent Prog Horm Res 56:359–375
Mathew SJ, Coplan JD, Schoepp DD, Smith EL, Rosenblum LA, Gonman JM (2001) Glutamate–hypothalamic–pituitary–adrenal axis interactions: implications for mood and anxiety disorders. CNS Spectr 6(555–556):561–564
Meister B (2007) Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav 92:263–271
Orlando G, Brunetti L, Di Nisio C, Michelotto B, Recinella L, Ciabattoni G, Vacca M (2001) Effects of cocaine- and amphetamine-regulated transcript peptide, leptin, and orexins on hypothalamic serotonin release. Eur J Pharmacol 430:269–272
Ottiger HP, Gerin-Moser A, Del Principe F, Dutly F, Streit P (1995) Molecular cloning and differential expression patterns of avian glutamate receptor mRNAs. J Neurochem 64:2413–2426
Reidiger T, Traebert M, Schmid HA, Scheel C, Lutz TA, Scharrer E (2003) Site-specific effects of ghrelin on the neuronal activity in the hypothalamic arcuate nucleus. Neurosci Lett 341:151–155
Riters LV, Bingman VP (1994) The NMDA receptor antagonist MK-801 impairs navigational learning in homing pigeons. Behav Neural Biol 62:50–59
Saito ES, Kaiya H, Tachibana T, Tomonage S, Denbow DM, Kangawa K, Furuse M, Furuse M (2005) Inhibitory effects of ghrelin on food intake is mediated by the corticotropin-releasing-factor system in neonatal chicks. Regul Pept 125:201–208
Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K (2001) Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 50:227–232
Stanley BG, Butterfield BS, Grewal RS (1997) NMDA receptor coagonist glycine site: evidence for a role in lateral hypothalamic stimulation of feeding. Am J Physiol 273:790–796
Taati M, Nayebzadeh H, Khosravinia H, Cheraghi J (2010) The role of the histaminergic system on the inhibitory effect of ghrelin on food intake in broiler chickens. IJVR 11(1):38–45
Tannenbaum GS, Lapointe M, Beaudet A, Howard AD (1998) Expression of growth hormone secretagogue-receptors by growth hormone-releasing hormone neurons in the mediobasal hypothalamus. Endocrinology 139:4420–4423
Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, Shioda S, Matsukura S, Kangawa K, Nakazato M (2003) Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 144:1506–1512
Tschop M, Statnick MA, Suter TM, Heiman ML (2002) GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology 143:558–568
Van den Pol AN, Trombley PQ (1993) Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci 13:2829–2836
Van Tienhoven A, Juhaz LP (1962) The chicken telencephalon, diencephalons and mesencephalon in stereotaxic coordinates. J Comp Neural 118:185–197
Zendehdel M, Baghbanzadeh A, Babapour V, Cheraghi J (2009) The effects of bicuculline and muscimol on glutamate-induced feeding behavior in broiler cockerels. J Comp Physiol A 195:715–720
Zeni LA, Seidler HB, De Carvalho NA, Freitas CG, Marino-Neto J, Paschoalini MA (2000) Glutamatergic control of food intake in pigeons: effects of central injections of glutamate, NMDA, and AMPA receptor agonists and antagonists. Pharmacol Biochem Behav 65(1):67–74
Acknowledgment
This research was supported by a grant from Research Council of the College of Veterinary Medicine, Lorestan University and a grant from Research Council of the Faculty of Veterinary Medicine, University of Tehran. Animal handling and experimental procedures were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication NO.85-23, revised 1996) and also with the current laws of Iran.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Taati, M., Nayebzadeh, H. & Zendehdel, M. The effects of DL-AP5 and glutamate on ghrelin-induced feeding behavior in 3-h food-deprived broiler cockerels. J Physiol Biochem 67, 217–223 (2011). https://doi.org/10.1007/s13105-010-0066-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-010-0066-y