Abstract
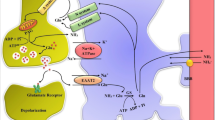
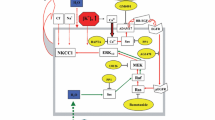
Astrocyte swelling (cytotoxic brain edema) is the major neurological complication of acute liver failure (ALF), a condition in which ammonia has been strongly implicated in its etiology. Ion channels and transporters are known to be involved in cell volume regulation, and a disturbance in these systems may result in cell swelling. One ion channel known to contribute to astrocyte swelling/brain edema in other neurological disorders is the ATP-dependent, nonselective cation (NCCa-ATP) channel. We therefore examined its potential role in the astrocyte swelling/brain edema associated with ALF. Cultured astrocytes treated with 5 mM ammonia showed a threefold increase in the sulfonylurea receptor type 1 (SUR1) protein expression, a marker of NCCa-ATP channel activity. Blocking SUR1 with glibenclamide significantly reduced the ammonia-induced cell swelling in cultured astrocytes. Additionally, overexpression of SUR1 in ammonia-treated cultured astrocytes was significantly reduced by cotreatment of cells with BAY 11–7082, an inhibitor of NF-κB, indicating the involvement of an NF-κB-mediated SUR1 upregulation in the mechanism of ammonia-induced astrocyte swelling. Brain SUR1 mRNA level was also found to be increased in the thioacetamide (TAA) rat model of ALF. Additionally, we found a significant increase in SUR1 protein expression in rat brain cortical astrocytes in TAA-treated rats. Treatment with glibenclamide significantly reduced the brain edema in this model of ALF. These findings strongly suggest the involvement of NCCa-ATP channel in the astrocyte swelling/brain edema in ALF and that targeting this channel may represent a useful approach for the treatment of the brain edema associated with ALF.








Similar content being viewed by others
References
Norenberg MD, Rama Rao KV, Jayakumar AR. Signaling factors in the mechanism of ammonia neurotoxicity. Metab Brain Dis. 2009;24:103–17.
Jayakumar AR, Norenberg MD. The Na-K-Cl Co-transporter in astrocyte swelling. Metab Brain Dis. 2010;25(1):31–8.
Walcott BP, Kahle KT, Simard JM. Novel treatment targets for cerebral edema. Neurotherapeutics. 2012;9:65–72.
Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–40.
Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, et al. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117:2105–13.
Ducis I, Norenberg LO, Norenberg MD. The benzodiazepine receptor in cultured astrocytes from genetically epilepsy-prone rats. Brain Res. 1990;531:318–21.
Juurlink BH, Hertz L. Plasticity of astrocytes in primary cultures: an experimental tool and a reason for methodological caution. Dev Neurosci. 1985;7:263–77.
Lange SC, Bak LK, Waagepetersen HS, Schousboe A, Norenberg MD. Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res. 2012;37:2569–88.
Kletzien RF, Pariza MW, Becker JE, Potter VR. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Ann Biochem. 1975;68:537–44.
Bender AS, Norenberg MD. Effect of benzodiazepines and neurosteroids on ammonia-induced swelling in cultured astrocytes. J Neurosci Res. 1998;54:673–80.
Jayakumar AR, Valdes V, Norenberg MD. The Na-K-Cl cotransporter in the brain edema of acute liver failure. J Hepatol. 2011;54:272–8.
Simard JM, Chen M. Regulation by sulfanylurea receptor type 1 of a non-selective cation channel involved in cytotoxic edema of reactive astrocytes. J Neurosurg Anesthesiol. 2004;16:98–9.
Hilgier W, Albrecht J, Kraśnicka Z. Thioacetamide-induced hepatic encephalopathy in the rat. I. Preliminary morphological and biochemical observations. Neuropatol Pol. 1983;21:487–94.
Zimmermann Z, Ferenci P, et al. Hepatic encephalopathy in thioacetamide induced acute liver failure in rats: characterization an improved model and study of amino acid-ergic neurotransmission. Hepatology. 1989;9:594–601.
Hilgier W, Olson JE, Albrecht J. Relation of taurine transport and brain edema in rats with simple hyperammonemia or liver failure. J Neurosci Res. 1996;45:69–74.
Rama Rao KV, Reddy PV, Tong X, Norenberg MD. Brain edema in acute liver failure: inhibition by L-histidine. Am J Pathol. 2010;176:1400–8.
Rashidy-Pour A. ATP-sensitive potassium channels mediate the effects of a peripheral injection of glucose on memory storage in an inhibitory avoidance task. Behav Brain Res. 2001;126:43–8.
Gammal SH, Basile AS, Geller D, et al. Reversal of the behavioral and electrophysiological abnormalities of an animal model of hepatic encephalopathy by benzodiazepine receptor ligands. Hepatology. 1990;11:371–8.
Jayakumar AR, Tong XY, Curtis KM, Ruiz-Cordero R, Abreu MT, Norenberg MD. Increased toll-like receptor 4 in cerebral endothelial cells contributes to the astrocyte swelling and brain edema in acute hepatic encephalopathy. J Neurochem. 2013. doi:10.1111/jnc.12516
Mans AM, Saunders SJ, Kirsch RE, Biebuyck JF. Correlation of plasma and brain amino acid and putative neurotransmitter alterations during acute hepatic coma in the rat. J Neurochem. 1979;32:285–92.
Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology. 1992;15:449–53.
Jayakumar AR, Panickar KS, Murthy CR, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci. 2006;26:4774–84.
Sinke AP, Jayakumar AR, Panickar KS, Moriyama M, Reddy PV, et al. NFkappaB in the mechanism of ammonia-induced astrocyte swelling in culture. J Neurochem. 2008;106:2302–11.
Campbell JD, Sansom MS, Ashcroft FM. Potassium channel regulation. EMBO Rep. 2003;4:1038–42.
Rama Rao KV, Jayakumar AR, Norenberg DM. Ammonia neurotoxicity: role of the mitochondrial permeability transition. Metab Brain Dis. 2003;18:113–27.
Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166:3873–81.
Delpire E, Days E, Lewis LM, Mi D, Kim K, Lindsley CW, et al. Small-molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A. 2009;106:5383–8.
Woo SK, Kwon MS, Geng Z, Chen Z, Ivanov A, Bhatta S, et al. Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J Cereb Blood Flow Metab. 2012;32:525–36.
Mao X, Chai Y, Lin YF. Dual regulation of the ATP-sensitive potassium channel by caffeine. Am J Physiol Cell Physiol. 2007;292:C2239–58.
Konopacka A, Konopacki FA, Albrecht J. Protein kinase G is involved in ammonia-induced swelling of astrocytes. J Neurochem. 2009;109:246–51.
Lin YF, Chai Y. Functional modulation of the ATP-sensitive potassium channel by extracellular signal-regulated kinase-mediated phosphorylation. Neuroscience. 2008;152:371–80.
Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29:317–30.
Chen BC, Lin WW. PKC- and ERK-dependent activation of I-kappa-B kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br J Pharmacol. 2001;134:1055–65.
Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush 3rd B, et al. Na–K–Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem. 2008;283:33874–82.
Pichili VB, Rao KV, Jayakumar AR, Norenberg MD. Inhibition of glutamine transport into mitochondria protects astrocytes from ammonia toxicity. Glia. 2007;55:801–9.
Elmalí E, Altan N, Bukan N. Effect of the sulphonylurea glibenclamide on liver and kidney antioxidant enzymes in streptozocin-induced diabetic rats. Drugs R D. 2004;5:203–8.
Acknowledgments
This work was supported by a Merit Review from the Department of Veterans Affairs and by the National Institutes of Health grant DK063311. We thank Alina Fernandez-Revuelta for the preparation of cell cultures.
Conflict of Interest
Arumugam Jayakumar declares no conflict of interest. He does not have a financial relationship with any organization.
Vanessa Valdes declares no conflict of interest. She does not have a financial relationship with any organization.
Xiao Ying Tong declares no conflict of interest. She does not have a financial relationship with any organization.
Nagarajarao Shamaladevi declares no conflict of interest. She does not have a financial relationship with any organization.
Will Gonzalez declares no conflict of interest. He does not have a financial relationship with any organization.
Michael Norenberg declares no conflict of interest. He does not have a financial relationship with any organization.
Human subjects were not involved in this study.
All institutional and national guidelines for the care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayakumar, A.R., Valdes, V., Tong, X.Y. et al. Sulfonylurea Receptor 1 Contributes to the Astrocyte Swelling and Brain Edema in Acute Liver Failure. Transl. Stroke Res. 5, 28–37 (2014). https://doi.org/10.1007/s12975-014-0328-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-014-0328-z