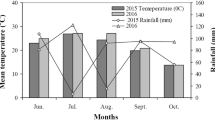
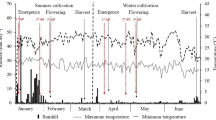
Abstract
The problem of declining tree yield has led to an investigation into the effectiveness of foliar application of exogenous hormones to improve flowering, fruit set, and fruit retention in cashew. Five exogenous hormones, one Gibberellic Acid (GA3) and four Auxins (IAA, IBA, NAA, and 2,4-D) at seven different rates of application (0 mg L−1, 10 mg L−1, 25 mg L−1, 50 mg L−1, 100 mg L−1, 250 mg L−1, and 500 mg L−1) were tested on six yield-related components of the two Brazilian cashew genotypes. This trial was a factorial split-split-plot design with each treatment replicated five times within a tree and three replications (three trees) per genotype. Responses varied significantly between exogenous hormones, concentrations and genotypes. The cashew plants used showed hormone-specific and optimum concentration response patterns. Of the five exogenous hormones tested, GA3 was most effective as its application at 50–100 mg L−1 gave five-fold improvements in flowering (precocity and number of hermaphrodite flowers) and fruiting, and about 69% increase in fruit retention ability and 25% in nut size. Panicles treated with GA3 also produced relatively bigger nuts compared to the untreated. Days to flowering was found to be hormone sensitive, while production of hermaphrodite flowers, fruit set, and nut development tended to be concentration specific. The GA3 exhibited a broad concentration tolerance among the five exogenous hormones investigated. Our data showed that using GA3 at 50 mg L−1 will enhance flowering precocity, shorten flowering duration, increase production of hermaphrodite flowers and fruit set significantly, and resultant nuts develop optimally with high percentage retention. Thus, it suggests cashew yield could be increased by exogenous foliar application of GA3 at 50–100 mg L−1 at pre-blooming stage.
Similar content being viewed by others
Abbreviations
- GA3 :
-
Gibberellic acid
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- NAA:
-
1-naphthaleneacetic acid
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
References
Aliyu OM. 2004. Characterization and compatibility studies in Cashew (Anacardium occidentale L). Ph.D Thesis, University of Ilorin, Nigeria. pp 266
Aliyu OM. 2006. Phenotypic correlation and path coefficient analysis of nut yield and yield related components in cashew. Silvae Genet. 55: 19–24
Aliyu OM. 2008. Compatibility and fruit-set in cashew (Anacardium occidentale L.). Euphytica 160: 25–33
Aliyu OM, Hammed LA. 2000. A study of nut and apple development in cashew (Anacardium occidentale L.). Nigerian J. Tree Crop Res. 4: 1–10
Aliyu OM, Awopetu JA. 2003. Studies on flowering pattern in cashew (Anacardium occidentale L.). Nigerian J. Genet. 18: 29–35
Aliyu OM, Awopetu JA. 2007. Multivariate analyses of cashew (Anacardium occidentale L.) germplasm in Nigeria. Silvae Genet. 56: 170–179
Blaikie S, O’Farrell P, Muller W, Wei X, Scott N, Skyes S, Chacko EK. 2002. Assessment and selection of new cashew hybrids. A Report for the Rural Industries Research and Development Corporation (RIRDC), Australia, RIRDC Publication No. 01/177, pp 21
Chacko EK, Baker I, Downton JWS. 1990. Towards a sustainable cashew industry for Australia. J. Aust. Inst. Agric. Sci. 3: 39–43
Chacko EK, Lu P, Kohli RR. 1995. Photoassimilate production and distribution in relation to productivity in mango (Mangifera indica L.). In: Mango 2000 — Marketing Seminar and Production Workshop Proceedings. Department of Primary Industries, Brisbane, pp. 109–124
Chalupka W. 1981. Influence of growth regulators and polythene covers on flowering of Scots pine and Norway spruce grafts. Silvae Genet. 30: 142–146
Chen WS. 1983. Cytokinins of the developing mango fruit. Plant Physiol. 71: 356–362
Chen WS. 1985. Flowering induction in mango (Mangifera indica L.) with plant growth substances. Proceedings National Science Council Part B, Life Sciences (Republic of China) 5: 49–55
Davenport TL. 2003. Management of flowering in three tropical and subtropical fruit tree species. HortScience 38: 1331–1335
Davenport TL. 2009. Reproductive physiology, In RE Litz, eds., The Mango: Botany, Production and uses, CABI, Oxford, pp 97–169
Davenport TL, Pearce DW, Rood SB. 2001. Correlation of endogenous gibberellic acid with initiation of mango shoot growth. J. Plant Growth Regul. 20: 308–315
Dokoozlian NK. 2001. Gibberellic acid applied at bloom reduces fruit set and improves size of ‘Crimson Seedless’ table grapes. Hortscience 364: 706–709
El-Otmani M. 1992. Principal growth regulator uses in citrus production. Proc.2nd Seminar on Citrus Physiology, Bebedouro Ed. Cargill. pp. 55–69
FAO. 2008. Food and Agriculture Organization of the United Nations, Crop Production Statistics Division. http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567. website last visited 19th June 2010
Foltan H, Ludders P. 1995. Flowering, fruit set and genotype compatibility in cashew. Ange. Bot. 69: 215–220
Fujioka S, Yamaguchi I, Murofushi N, Takahashi N, Kaihara S, Takimoto A. 1983. The role of plant hormones and benzoic acid in flowering of Lemna aucicostata 151 and 381. Plant Cell Physiol. 24: 241–246
Gawankar MS, Sawale RD, Pawar SN, Chavan SA. 2010. Effect of Ethrel® on flowering, sex-expression and yield in cashew. J. Hort. Sci. 51: 68–70
Ghosh SN. 1989. Effect of nitrogen, phosphorus and potassium on flowering duration, yield and shelling percentage of cashew (Anacardium occidentale L.). Indian Cashew J. 19: 19–23
Heard TA, Vithanage V. and Chacko EK. (1990). Pollination biology of cashew in the Northern Territory of Australia. Aust. J. Agric. Res. 41: 1101–1114
Kachru RB, Singh RN, Chacko EK. 1972. Inhibition of flowering in Mangifera indica L. by gibberellic acid. Acta Hort. 24: 206–209
Khan NA, Mobin M, Samiullah M. 2004. The influence of gibberellic acid and sulfur fertilization rate on growth and S-use efficiency of mustard (Brassica juncea). Plant Soil 270: 269–274
Kulkarni VJ. 2004. The tri-factor hypothesis of flowering in mango. Acta Hort. 645: 61–70
Kurian RM, Iyer CPA. 1993. Chemical regulation of tree size in mango (Mangifera indica L.) cv. Alphonso. III. Effects of growth retardants on yield and quality of fruits. J. Hort. Sci. 68: 361–364
Martins PJ, Kasuga LJ. 1995. Variation in cashew tree yield in Southeast Tanzania and the implication for management of cashew smallholder farmers in Tanzania. Exp. Agric. 72: 261–268
Masawe PAL, Cundall EP, Caligari PDS. 1996. Distribution of cashew flower sex-types between clones and sides of tree canopies in Tanzania. Ann. Bot. 78: 553–55.
Murthy KN, Yadava RBR, Bigger J. 1975. Increasing fruit set in cashew by hormone spraying. J. Plantation Crops (India), 3: 81–82
Nordstrom A, Tarkowski P, Tarkowska D, Norbaek R, Astot C, Dolezal K, Sandberg G. 2004. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc. Natl. Acad. Sci. USA 101: 8039–8044.
Nunez-Elisea R, Davenport TL. 1983. Abscission and ethylene production of mango (Mangifera indica L.) fruit cv. Tommy Atkin. Proc. Florida State Hort. Soc. 96: 185–188
Nunez-Elisea R, Davenport TL. 1998. Gibberellin and temperature effects on dormancy release and shoot morphogenesis of mango (Mangifera indica L.). Sci. Hort. 77: 11–21
Ohler JG. 1979. Cashew. Koninklijk instituut voor de Tropen. Teskin, Zutphen Co. Amsterdam, The Netherlands
Parameswaran NK, Daramodaran VK, Prabhakaran PV. 1984. Factors influencing yield in cashew (Anacardium occidentale L). Indian Cashew J. 16: 15–19
Patnaik HP, Das MS, Panda JM. 1985. Studies on fruit set and fruit drop in cashew (Anacardium occidentale L.) under Orissa conditions. Cashew Causerie 7: 7–8
Ram SL, Bist D, Lakhanpal SC, Jamwal IS. 1976. Search of suitable pollinizers for mango cultivars. Acta Hort. 57: 253–263
Ramming DW, Tarailo R, Badr SA. 1995. ’Crimson Seedless’: A new late maturing, red seedless table grape. Hortscience 30: 1473–1474
Rao VNM. 1956. Multiply the better yielding cashew nut. Indian Farming (India). 6: 19–23
Roitsch T, Ehneb R. 2000. Regulation of source/sink relations by cytokinins. Plant Growth Regu. 32: 359–367
Schuch UK, Fuchigami HL, Nagao MA. 1990. Gibberellic acid causes earlier flowering and synchronizes fruit ripening of coffee. Plant Growth Regul. 9: 59–64
Searle C, Whiley AW, Simpson DR, Saranah JB. 1995. A preliminary phenol-physiological model for ‘Kensington’ mango in subtropical Australia. In Mango 2000-Marketing Seminar and Production Workshop Proceedings. Department of Primary Industries, Brisbane, pp. 109–124
Subbaiah CC. 1983. Fruiting and abscission patterns in cashew. J. Agric. Sci. (Cambridge) 100: 423–427
Topper CP, Caligari PDS, Camara M, Diaora S, Djaha A, Coulibay F, Asante AK, Boamah A, Ayodele EA, Adebola PO. 2001. Report of the West African regional cashew survey covering Guinea, Guinea Bissau, Cote d’Ivore, Ghana and Nigeria. Sustainable Tree Crop Programme (STCP) Report No. BHA-01109. Biohybrids Agrisystem Ltd. P. O. Box 2411, Earley, Reading RG6 5FY, UK
Weaver RJ. 1958. Effect of gibberellic acid on fruit set and berry enlargement in seedless grapes of Vitis vinifera. Nature 181: 851–852
Wright G. 2004. Plant growth regulators used in Citrus production. http://ag.arizona.edu/crops/presentations/2004/wrightpgrho051204.pdf An unpublished presentation to the University of Arizona, USA. Website last visited 19th June 2010
Wunnachit W, Sedgley M. 1992. Floral structure and Phenology of cashew in relation to yield. J. Hort. Sci. 67: 769–777
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aliyu, O.M., Adeigbe, O.O. & Awopetu, J.A. Foliar application of the exogenous plant hormones at pre-blooming stage improves flowering and fruiting in cashew (Anacardium occidentale L.). J. Crop Sci. Biotechnol. 14, 143–150 (2011). https://doi.org/10.1007/s12892-010-0070-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-010-0070-3