Abstract
Genetics has traditionally enabled the reliable diagnosis of patients with rare genetic disorders, thus empowering the key role of today’s clinical geneticists in providing healthcare. With the many novel technologies that have expanded the genetic toolkit, genetics is increasingly evolving beyond rare disease diagnostics. When placed in a transition context—like we do here—clinical genetics is likely to become a fully integral part of future healthcare and clinical genetic expertise will be required increasingly outside traditional clinical genetic settings. We explore transition effects on the thinking (culture), organizing (structure), and performing (practice) in clinical genetics, taking genetic healthcare in Estonia, Finland, and the Netherlands as examples. Despite clearly distinct healthcare histories, all three countries have initially implemented genetic healthcare in a rather similar fashion: as a diagnostic tool for predominantly rare congenital diseases, with clinical geneticists as the main providers. Dynamics at different levels, such as emerging technologies, biobanks and data infrastructure, and legislative frameworks, may require development of a new system attuned with the demands and (historic) context of specific countries. Here, we provide an overview of genetic service provisions in Estonia, Finland, and the Netherlands to consider the impact of historic and recent events on prospective developments in genetic healthcare.
Similar content being viewed by others
Introduction
Clinical genetics is an established healthcare service dedicated to the study, treatment, and counseling of individuals with heritable diseases and disorders predisposition. New technologies and methodologies like next-generation sequencing (NGS) technologies, genome-wide association studies (GWAS), and the Clustered Regularly Interspaced Short Palindromic Repeats-associated nuclease 9 (CRISPR/Cas9) system are rapidly expanding the genomic toolkit and increasing the diagnostic power, risk assessment, and treatment options for many diseases, beyond rare disorders, and outside of the traditional boundaries of clinical genetics. While the focus is often on the potential of these technologies for solving persistent challenges for rare diseases—e.g., optimizing diagnosis and characterization—we aim here to explore how such emerging technologies affect the context in which clinical genetics operates.
Healthcare systems are under increasing demands from a changing society and face rising costs, growing fragmentation, and staff reduction (Johansen et al. 2018). Globally, inequalities are widening, the population is aging, and individualism (prioritizing personal interests over those of the wider group) is increasing (Kerry et al. 2017; Santos et al. 2017). At the same time, innovation and new technological developments are continually translated into practice, creating new opportunities for diagnosis, treatment, and care. Medical innovations often inspire their developers to reflect on potential healthcare transformations (Haghi et al. 2017; Hamet and Tremblay 2017; Rus and Tolley 2015; Stark et al. 2019); however, the reality is that the dynamics of healthcare transitions are generally more complex than anticipated.
Emerging technological and societal developments warrant a reconsideration of how clinical genetic services are provided (Battista et al. 2012; Unim et al. 2019). Future genetic healthcare is often envisioned as more proactive—personal, preventive, participatory—and less reactive—diagnose-and-treat (Veltman et al. 2013). Identifying individuals with a high genetic risk for chronic diseases is expected to help target preventive measures, thus postponing or even preventing disease cases and improving quality of life. Crucially, this would also significantly reduce societal and healthcare costs (Inouye et al. 2018; Stark et al. 2019). However, these kinds of proactive genetic-based models have difficulty setting foot in the current healthcare landscape. Various challenges have been identified (e.g., professional protectionism, suboptimal levels of professional genetic knowledge and skills, high standards for evidence, and need for (data-)infrastructure and resources), which could be collectively described as a “lack of preparedness of the healthcare system” (Battista et al. 2012; Martin et al. 2009; Unim et al. 2019).
Putting genetic developments into a transition management context may help in understanding how the changes within healthcare systems could impact the culture, structure, and practice of clinical genetics (Rotmans et al. 2001; Geels and Schot 2010; Loorbach et al. 2017; Rotmans 2005; van Raak 2016; Wittmayer et al. 2017). Typically, transition management is aimed at responsible implementation of change through systematic planning, organizing, and application of key steps in desired transitions. To study key steps of transitions, (aspects of) transition theory can be applied, enabling understanding of the current situation and expected and desired actions. Transition theory has been successfully applied to unravel, structure, and support transitions in other societal systems, such as energy, mobility, and waste transitions (Loorbach et al. 2017). Moreover, a transition-based framework has previously been used to analyze and understand key barriers and facilitators for innovation in specific genetic services (Holtkamp et al. 2017; Rigter et al. 2014). Here, we utilize a transitional framework to consider the impact of historic, recent, and future developments in genetic healthcare.
In the context of this JoCG special issue on rare diseases, we focus on the fields that are currently most relevant for rare disease genetics and where changes in service delivery are observed or expected, i.e., clinical genetics, public health genomics, and genetic research. In the remainder of this contribution, we collectively refer to these as “genetic healthcare.” We first describe the development and key characteristics of genetic healthcare in three countries—Estonia, Finland and, the Netherlands—all of which are dedicated to investing in innovation in healthcare, but differ in the organization and funding of their (genetic) healthcare services (Keskimaki et al. 2019; Kroneman et al. 2016; Lai et al. 2013; Postelnicu 2019). We will subsequently identify the similarities and differences of the clinical genetic regimes in the three countries, characterize key elements of culture, structure, and practice in clinical genetics, and envision how this may influence future developments.
A multi-level perspective on the origin and development of genetic services in Estonia, Finland, and the Netherlands
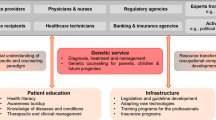
Following classic transition management theory for socio-technical regimes, we consider genetic healthcare from a multi-level perspective, aiming to unravel and characterize the autonomous developments (“landscape”), the system-specific configuration (“regime”), and the emerging innovations (“niches”) that shape genetic healthcare (Rotmans et al. 2001). Our focus is on the particular configurations in which genetics serves as an application. We will characterize the genetic healthcare regimes as configurations of the elements culture, structure, and practice (Rigter et al. 2014; van Raak 2016). In this context, the national healthcare system may be considered “landscape,” while clinical genetics, public health genomics, and genetic research may serve as distinct “regimes.” Developments in sequencing technologies, biobanking, and data regulation would then be examples of emerging “niches.” An overview of how we apply transition theory is depicted in Fig. 1.
Transition theory. True integration of innovation in (public) healthcare requires different phases in transition and different levels of structuration. While dynamics in the healthcare system (here considered the landscape) impact culture, structure, and practice of relative autonomous genetic regimes, relevant niche developments likely will push towards transition, e.g., by broader implementation, increasing collaboration, and harmonization and structuration of initiatives (adapted from: Rigter et al. 2020)
Globally, genetic healthcare emerged in the 1970s following the scientific breakthroughs in the late nineteenth century and the mid-1900s (Hardy 1908; Mendel 1941; Tjio and Levan 1956; Weinberg 1909). How genetic services subsequently developed was predominantly based on the existing healthcare infrastructures shaped by different legislative frameworks. To gain more insight into the impact of nation-specific historical landscapes on current clinical genetic practices, three distinct European countries were selected: Estonia as a Baltic state and a previous member of the Soviet Union, Finland as a representative of the Northern European or Scandinavian countries, and the Netherlands as a representative of western Europe. By the 1970s, healthcare in Finland and the Netherlands had matured into accessible, highly regulated, and government supported systems, while general access and financing of healthcare was limited in Estonia—then still a Soviet republic. To provide insight into the landscapes, we first highlight the relevant national policies, regulations, and resources within the three healthcare systems that have predominantly shaped the regimes or models for delivery of genetics services in these countries (Battista et al. 2012; Unim et al. 2019). Furthermore, we describe the national clinical genetics, public health genomic, and genetic research regimes for each country.
Estonia
Estonia regained independence from the Soviet Union in 1991 and its current healthcare system landscape therefore is considered relatively young (Optimity Advisors 2018). Like the other Baltic states (Latvia and Lithuania), Estonia initiated heavy reforms to break with the centralized, inefficient, and low-quality “Semashko” heritage for healthcare (Sheiman et al. 2018; van Ginneken et al. 2012). Estonia’s government made family medicine a specialty with corresponding postgraduate training (strengthening of primary care), improved the technical proficiency of hospitals (capacity building), and performed a rapid transition from budget-based healthcare to centralized solidarity-based insurance healthcare backed by the Estonian Health Insurance Fund (insurance medicine) (Lai et al. 2013). Healthcare in Estonia is predominantly centrally funded from a proportional flat rate social tax paid by employers. Two reforms—broad scale digitalization in healthcare and the hospital reform—have made the Estonian healthcare systems one of the most progressive in the world (van Ginneken et al. 2012). The focus on technical proficiency in hospitals coincided with the government’s ambition to make Estonia a largely digitalized society. A key outcome of this convergence was the nationwide introduction of Electronic Health Records in 1998, the first country in the world to do so (Tiik and Ross 2010). A number of parallel initiatives—e.g., e-citizenship, e-governance, and e-voting—have further boosted the digital infrastructures and general participation of citizens. Personal use and access of electronic health records by citizens in Estonia is currently the highest rate in Europe (Ćwiklicki et al. 2020). Centrally managed E-Health Database includes Healthcare Imaging Database (established 2006), e-Prescriptions (2010), and e-Registration (2019). Ninety-nine percent of prescriptions are digital and 100% healthcare billing is digital (e-Health Records — e-Estonia (e-estonia.com) n.d.).
The Estonian government is also investing in the realization of personalized medicine at the national level, with an initial focus on pharmacogenomics and application of genetic testing for common complex disorders. For this purpose, there is increased attention to more structured databases, real-time and customized access to health data, and medical decision-support systems (Lai et al. 2013). Related updates to the electronic health records have been announced (Meditsiini Uudised 2020).
The clinical genetics regime in Estonia is largely centralized; two clinical genetic departments in Tallinn and Tartu provide access to advanced genetic counseling and cascade screening. Since the end of 1990s, clinical genetics training in Estonia has had its own curriculum, separate from other medical specialties. In general, clinical geneticists work with a broad spectrum of suspected genetic disorders, from rare pediatric problems to more frequent familial cancer syndromes or risk factors for, e.g., thrombophilia. Specialists outside of clinical genetics, for example neurologists, have a long tradition of directly requesting genetic testing for their patients independent of clinical geneticists. In line with centralized clinical genetics services, genetic testing in Estonia is mostly provided by Tartu University Hospital, although genetic testing can also be performed by private laboratories. Clinicians are free to choose service providers. Clinical genetic service is fully reimbursed by Estonian Health Insurance Fund (EHIF), with the volume depending on the allocated budget for specific clinical service providers. Genetic services outside Estonia are also covered but on a case basis via a specific application process, if such services are not available in Estonia.
Maternity and child care services, including aspects of the public health genetic care regime—e.g., newborn and prenatal screening—are free of charge, as is the national vaccination program for 12 recommended diseases and cancer screening programs. Neonatal screening covers neonatal hypothyreosis, and 20 treatable metabolic disorders and it is offered to all children since all children automatically have a health insurance (Mikselaar et al. 1998; Reinson 2018). Breast and ovarian cancer screening as well is centrally funded, through the EHIF and coordinated by the Cancer Screening Foundation. Starting from 2021, public cancer screening programs are freely available for all Estonian residents independent of their health insurance status (BNS/TBT Staff (Baltictimes.com) 2020).
The genetic research regime in Estonia is profiting from the large genetic dataset that is available in the country. The Estonian Biobank has invested in recruitment and integration strategies which have produced a robust biobank currently representing over 20% of the adult population (Leitsalu et al. 2014). All participants in the Estonian biobank (>200,000) have been genotyped, and a considerable part has been sequenced (5000 exomes and whole genomes). Selected findings (actionable high-risk findings, polygenic risk scores, pharmacogenetics data, etc.) are offered to participants included in various studies. Data use and privacy protection of the biobank, which is linked to data from the Electronic Health Records, is largely regulated by the Estonian Human Genes Research Act (implemented in 2000) and the European General Data Protection Regulation (GDPR) (Keis 2016). The Human Genes Research Act aims to, e.g., ensure the voluntary nature of gene donation and the confidentiality of the identity of gene donors, and to protect persons from misuse of genetic data and from discrimination based on interpretation of the structure of their DNA and the genetic risks arising therefrom. The GDPR is aimed at the protection of natural persons with regard to the processing of personal data and on the free movement of such data in general.
Finland
The Finnish healthcare landscape reflects the Nordic tradition of investment in the quality, equality, and solidarity of healthcare, and provides comprehensive (public) healthcare services for all inhabitants, as regulated by the Constitution (Ahola-Launonen 2016; Keskimaki et al. 2019). Healthcare in Finland is largely financed by taxes (municipalities), insurance fees (National Health Insurance, NHI), and joint employer-employee premiums (occupational healthcare). Publicly funded healthcare is complemented by a growing private healthcare industry due to the increasing demand for various healthcare services (Keskimaki et al. 2019; Finnish Ministry of Social Affairs (Private health care - Sosiaali- ja terveysministeriö (stm.fi) (n.d.)).
The introduction of a clinical genetic regime in the 1970s was driven by the convergence of two phenomena (Norio et al. 1973; Reijo Norio 2003b). First, there was increasing evidence that particular rare hereditary diseases were overrepresented in Finland—the so-called Finnish Disease Heritage (FDH) (Reijo Norio 2003a). This led to extensive research on the natural history, treatment, and genetic background of nearly 40 monogenic diseases prevalent in the country (Findis (The Finnish Disease Database: FinDis.org) n.d.). Second, university hospitals founded cytogenetic laboratories, providing genetic diagnostic services for patients with rare diseases (Von Koskull and Salonen 1997). Today, clinics primarily purchase tests from their own university hospitals, with the flexibility to turn to laboratories elsewhere (Finland or Europe—both academic and private) in case of unavailability (Pohjola et al. 2016). Ordering and/or explaining results of genetic tests are not regulated in Finland except that, as for all tests, they have to be ordered by a healthcare professional. The focus of clinical genetics has been on diagnosis and counseling of patients with rare diseases, but the demand for genetic services for more common diseases such as familial cancer is growing. Clinical geneticists focus on diagnostics and cascade screening. Often, the diagnostic tests have already been done by other specialties and the patient is referred for help in interpreting the results, counseling, and family member testing.
As for the genetic research regime, human genetic studies in Finland are increasingly facilitated through biobanks. The Finnish Biobanks comprise samples, health information, and genomic data from over 400,000 donors (Finngen (Finngen research project) n.d.). Legislation is largely focused on the collection and use of genetic material within these biobanks, predominantly through the Biobank Act. This legislation is soon expected to be supplemented with the Genome Act, which is intended to enable the use of genomic biobank data in personal healthcare and prevention (Soini 2016)
A hallmark of Finish healthcare—as for other Nordic countries—is high-quality national health registries which provide a solid basis for the national public healthcare regime. The registries are established and regulated by specific laws; no consent is required to add personal data to these registries, but access to the data is guarded by detailed processes for all stakeholders.
Many public health services are provided as national programs, largely directed towards health promotion and disease prevention (Healthcare in Finland 2013). These are generally nationally defined, funded, and coordinated while organized at the level of municipalities, comprising various vaccinations and screening programs, including fetal and neonatal screening, as well as maternity and child healthcare. A unique feature emphasized in The Primary Health Care Act (1999) is to mandate the municipalities to take health into account as part of other policies, for instance community planning, and to be responsible for disease prevention and health promotion (Eloranta and Auvinen 2015; Keskimaki et al. 2019). Furthermore, patients and patient organizations play a key role in shaping the genetic services in Finland. Patient organizations actively collaborate with the national healthcare system, offering extra services like organized peer support, rehabilitation services, and specific information on rare diseases (Von Koskull and Salonen 1997). The appreciation for genetic research is also reflected in the public attitude. Patients are aware of their rights (defined in legislation for 30 years) and usually very positive towards population level approaches and medical research (Borodulin et al. 2018).
The Netherlands
In a transition context, healthcare in the Netherlands is offered in the landscape of a predominantly curative system, which has developed from the constitutionalizing of the monarchy in 1848—and consequential shift of power (also of healthcare) to Parliament and (local) governments. It is comprised of various autonomously evolving regimes (e.g., the “medical specialists”) (van Raak and de Haan 2018). One of the main characteristics of the Dutch system is the gatekeeping principle, whereby hospital and specialist care require referral from a primary care provider such as a general practitioner, midwife, or dentist, with the exception of emergency care. The 2006 healthcare reforms introduced a market-oriented healthcare system, allowing citizens to freely choose insurer and healthcare provider, while obligating insurers to accept all applicants for the basic benefit package (set by the government), and compete on price and contents of the premium packages (Maarse et al. 2016). Healthcare providers likewise compete for patients and negotiate with insurers on services and fees. The basic health insurance package and compensations for lower incomes protect citizens against catastrophic spending. Out-of-pocket payments are low from an international perspective (Kroneman et al. 2016).
As for the clinical genetic regime, clinical genetics has been recognized as a medical specialty since 1987 (Battista et al. 2012). Accordingly, the Ministry of Health has appointed nine dedicated “clinical genetic centers” embedded in academic hospitals, installed legislation prohibiting non-academic hospitals and private companies from providing clinical genetic services (the Specialist Medical Practice Act), and included clinical genetic testing and counseling in basic health insurance (Borry et al. 2012; Nelis 1999). As such, clinical genetics was positioned into a tertiary care service model, evolving into a federated public, mainly diagnostic, support platform for secondary medical specialties such as pediatrics, cardiology, neurology, and oncology (Niermeijer 2011). The strong regulatory and financial embedding of clinical genetics in the Netherlands, however, has created a precedent for Dutch clinical geneticists to be the “treating physician” for their patients (hence often requiring referral from primary or secondary care professionals) at least until the end of the diagnostic odyssey and/or referral to secondary/primary care for treatment. Similarly, the clinical genetics centers have in-house laboratories which perform the majority of genetic testing. Relatively recently, like in Finland, other secondary medical specialties (e.g., oncology) are increasingly ordering genetic tests from the clinical genetic laboratories for their patients, for diagnostics, and personalized treatment plans.
The public healthcare regime in the Netherlands is mostly a nationally coordinated healthcare service comprising vaccination, environmental safety, and lifestyle intervention programs (Jambroes et al. 2013). National public health programs in the Netherlands are upon directive from the Ministry of Public Health, Welfare, and Sports (VWS). Scientific insights have traditionally informed public health policy and provision, with patient organizations regularly fueling debate about hypes and hopes of innovations. The national public health services provide various population screening programs (e.g., prenatal, neonatal, breast cancer) and a national immunization program. Besides the national services, public health is delivered as prevention programs at the municipal (for selective and indicated prevention of, e.g., diabetes and obesity) and/or primary care level (for collective prevention of, e.g., cardiovascular risk), but with little attention to genetic factors (Dutch National Institute for Public Health and the Environment (2010); Dutch National Institute for Public Health and the Environment (2021)).
Fueled by the 1989 Health Council report on genetic diagnostics and gene therapy—which included a recommendation to increase the “knowledge and insight in hereditary disease”—the clinical genetics community in the Netherlands started building a solid research infrastructure, resulting in the discovery of (additional) genetic causes for many rare congenital syndromes and the unravelling of novel disease mechanisms. These in turn have fueled the development of therapeutic strategies, and the launch of various large-scale population studies (Aartsma-Rus et al. 2009; Boomsma et al. 2014; Health Council of the Netherlands 1989; Gilissen et al. 2014; Harakalova et al. 2012; Hofman et al. 2007; Hoischen et al. 2010; Rook et al. 1999; Ropers and Hamel 2005; Scholtens et al. 2014; Smeets et al. 1992; Tessadori et al. 2018; Verkerk et al. 1991; Vissers et al. 2004). A number of ongoing, mainly local, cohort studies have recently started collecting and analyzing genetic data from their participants (Boomsma et al. 2014; Hofman et al. 2007; Scholtens et al. 2014).
Most healthcare providers in the Netherlands use a form of electronic patient records. The national roll-out of an electronic patient record system to interconnect these practice-based systems has not yet succeeded, mainly for reasons of privacy. Collecting and studying clinical, including genetic, data is challenging because of the lack of harmonization between the different systems, both from clinical and study cohorts (Kroneman et al. 2016). Recent initiatives aim to improve the findability, accessibility, interoperability, and reusability (the FAIR-data principle) of genetic data in the Netherlands (Zorginstituut Nederland, 2018).
Characterizing the regimes of genetic healthcare—culture, structure, and practice
Developments in landscapes have largely determined the characteristics of the current regimes of clinical genetics, public health genomics, and genomic research in Estonia, Finland, and the Netherlands. Here, we provide a more in-depth analysis of the similarities and differences of the clinical genetic regimes in these three countries in terms of the key elements of culture (how we think), structure (how we organize), and practice (what we do) (Table 1).
Culture
Traditionally, the purpose of genetic healthcare (particularly clinical genetics) has been to provide diagnoses for predominantly rare diseases. However, in both Estonia and Finland, genetic diagnostics have been considered part of the tool set of medical specialists other than clinical geneticists (e.g., pediatricians). This aspect of culture, namely the vision on the main application of genetics for diagnostics and perceived need to confine genetic services to the clinical genetic specialty, may affect attitudes towards adoption of emerging new technologies (niches) beyond the traditional boundaries of clinical genetics. For instance, the first attempts to use results from research on biobank samples in preventive healthcare have already been undertaken in Estonia, e.g.. return of copy number variants and actionable monogenic findings related to familial hypercholesterolemia and hereditary breast and ovarian cancer (Alver et al. 2019; Leitsalu et al. 2016, 2020). By the end of 2019, over 2500 participants had received a personal report with polygenic risk scores on type 2 diabetes, cardiovascular disease, early onset menopause, thrombophilia, hypolactasia, and glaucoma, as well as pharmacogenetics data. In Finland, similar application of polygenic risk scores to health promotion is being piloted in research settings (Marjonen et al. 2020). Some local genomic initiatives have only just initiated in The Netherlands, to apply broader genomic pre-symptomatic testing beyond the setting of clinical genetics (Sedaghati-Khayat et al. 2020),(Amsterdam UMC, Locatie VUmc - Mijn DNAmedicatiepas n.d.).
There is an ongoing debate on the information generated and reported from diagnostic studies in clinical genetic services. Genetic laboratories in Europe have traditionally followed the recommendations of the European Society of Human Genetics to apply targeted approaches in order to minimize incidental or secondary findings (Isidor et al. 2019; Matthijs et al. 2016; Vears et al. 2018). There is an increasing call for applying broader approaches for reasons of cost-effectiveness, opportunities to re-analyze or re-interpret (Sun et al. 2015), or as contributions to research (van El et al. 2013). Also, the opportunity to utilize data generated from high throughput sequencing to inform patients about “actionable variants” beyond the primary clinical inquiry (often referred to as “opportunistic screening”) is highly debated. The European Society of Human Genetics remains reluctant in this context and recommends a “cautious approach” (de Wert et al. 2020). Clinical genetic centers in Europe therefore still tend to prefer targeted interpretation approaches (Pajusalu et al. 2018; Vrijenhoek et al. 2015; Weiss et al. 2013). Conversely, the philosophy behind the Estonian Biobank has always been to collect the broadest possible genotypic data into a population biobank and use these data for healthcare whenever appropriate (Leitsalu et al. 2015). In Finland, biobanks linked with national healthcare registries have been collected to form a powerful national infrastructure for research and the use of Biobank data also in healthcare and health promotion is being formalized.
Structure
Clearly, clinical genetics has been the main regime for genetic healthcare applications in all three countries. However, organization differs considerably. For instance, in the Netherlands, the nine clinical genetic centers are autonomous units, devoid of competition, with essentially all necessary resources (finance, infrastructure, staff) available in-house. Dutch law prevents non-academic hospitals and private industry from conducting genetic services, while in Estonia and Finland, these roles are less confined. In Estonia and Finland, clinical genetic services offer genetic counseling and use both the hospitals’ as well as external (including foreign) laboratories for genetic testing, services increasingly also requested by non-genetic medical specialists. Besides the generally recognized medical profession of clinical geneticists in most countries in Europe, the three countries described here differ in their training and deployment of professionals specifically trained to conduct genetic counseling: genetic counselors. While Estonia and Finland lack specific training programs for this profession, Finland does have nurses trained in genetics. The Netherlands has several training programs and over 50 genetic counselors (Abacan et al. 2019).
In all three countries, there are indications of an increasing burden on clinical genetics staff and budget, predominantly as a result of increasing demand (Lynch and Borg 2016). At present, there are dedicated, often registered, clinical genetics staff (i.e., clinical geneticists, laboratory specialists, or genetic nurses and/or counselors) in all three countries (Lynch and Borg 2016). However, based on growing demand and waiting times for the clinics, there appears to be a general need for more genetically literate medical staff. Central organization—such as in Estonia—may require a relatively small staff. In Finland, long geographical distances require clinics in many parts of the country, and thus a considerable staff even in the sparsely populated northern and eastern parts. The federated clinical genetic regime (centrally coordinated, but with distributed responsibilities) in the Netherlands, with clinical genetics as an established department in each of the eight academic hospitals—thus boosting the demand for clinical genetic services from other medical specialists—would probably need the most increase in staff. A revolutionary change in the working traditions from comprehensive face-to-face counseling to, e.g., (online) tutorials supplemented, when needed, with short counseling sessions would radically decrease the need for more personnel. On the other hand, clinical geneticists or genetic counselors/nurses are increasingly needed as consultants and advisers outside the clinical genetic centers, as the use of genetic information expands in clinical practice and only patients with complex findings are referred to clinical geneticists. Still, clinical geneticists are the only specialty systematically engaging family members for cascade screening.
Whereas most of the legislation for clinical genetics is country-specific, the recent introduction of European legislation for the protection of natural persons with regard to the processing of personal data and on the free movement of such data (GDPR) has initiated reflections on the status, management, and use of genetic data across Europe. The effect of this European data uniformization effort has been different across countries. In Finland and Estonia, the traditional “broad consent model” in use when collecting biobank samples, or “no consent” relating to Finnish national healthcare registries, are being complemented by strict regulation of data access and the rights of donors/patients in specific national legislation, to be in line with GDPR (Soini 2016). Conversely, in the Netherlands, legislation around personal data traditionally seeks extensive control and assurance, e.g., through detailed consent procedures. The explicit procedures in the Netherlands on secondary use of data, unsolicited findings, and re-contacting therefore generally facilitate GDPR implementation, despite the variety of consent procedures across the country (Vrijenhoek et al. 2015). All other aspects of genetic services in the Netherlands are regulated by a patchwork of laws, codes of practices, and other ethical instruments, such as the Act on Population Screening (Wbo). These are converted into guidelines by national professional organizations, e.g., for clinical geneticists and for genetic laboratories (Vereniging Klinisch Genetische Laboratoriumdiagnostiek n.d.; Vereniging Klinische Genetica Nederland n.d.).
The absence of equivalents to the Dutch Specialist Medical Practice Act in Finland and Estonia (and in many other countries in Europe) more easily allows not only other specialists but also various local health centers, regional hospitals, and private clinics to offer genetic services. This potentially facilitates more efficient implementation of new genetic innovations outside the traditional clinical genetic regime, such as pre-symptomatic (pharmacogenomics) testing and liquid biopsy screening in cancer patients for early diagnosis or personalized treatment.
While biobanks and healthcare data in Estonia and Finland are already largely centralized in national registries, the Netherlands is only recently trying to make fragmented genetic data findable, accessible, interoperable, and reusable (FAIR) (Radboud 2018; Wilkinson et al. 2016).
Practice
In terms of the services delivered in the clinical genetic regimes, there seems to be general agreement on the essential components of clinical genetic care for patients in all three countries. For rare diseases, typically a primary or secondary care specialist refers the patient to genetic specialists, detailed phenotyping and clinical evaluation, pre- and post-test counseling, and possibly cascade screening (Vears et al. 2018). Genetic services for more common variants or diseases (e.g., polygenic risk scores) are generally considered to be (or become) part of routine primary or secondary care. Effective implementation in this setting, however, would require a redesign of these current regimes to meet essential prerequisites for good genetic care. This would include more emphasis on medical decision-making, formulating best practices on communicating genetic results and their consequences, and arranging of reimbursement. This is especially true for the countries where, as in the Netherlands, clinical genetic services have traditionally been mainly confined to the clinical genetic centers and clinical genetic specialists.
Since the introduction of high throughput sequencing techniques, the possibility of wider analysis of genomic data in research and clinical care became feasible, and ongoing decreasing cost trends are driving ever-wider implementation. At the present, in the clinical genetic regimes, a shift from a specific test to confirm a clinical diagnosis is seen towards broader tests such as gene panels or whole exome sequencing. This has given rise to several ethical and practical dilemmas; for some time already, the clinical genetics community is struggling to reach a consensus on proper use, including the appropriate model that comprehensively covers consent, informs family members, and provides directions for re-contacting and use of genetic data for research (van El et al. 2013; Vears et al. 2018). Although the European Society of Human Genetics provides recommendations on some of these aspects, practices are generally not harmonized at the national level, let alone the European level.
New service delivery models within the clinical genetics’ regime were quickly and broadly implemented in all three countries during the first SARS-Cov2 lockdowns in 2020. E-counseling replaced in-person counseling where needed, and online tutorials and educational materials were used to increase efficiency of information provision. Many guidelines and recommendations for new service delivery models have been published (e.g., on individual face-to-face counseling versus e-information, testing methods, returning results from biobanks, incidental, and secondary findings (Battista et al. 2012; Isidor et al. 2019; Sun et al. 2015; Vears et al. 2018)), but it is unclear what the actual uptake and (especially mid and long term) effects will be.
Population (cancer, neonatal, and prenatal) screening programs are implemented in the public health regimes of all three countries, while to a differing extent, pre-symptomatic screening for other hereditary disorders is implemented, piloted, or studied through the biobanks or other research infrastructures. While Estonia and Finland are inclined to do this at national level, the first broader applications of, e.g., pharmacogenetics or polygenic risk scores in the Netherlands is currently still restricted regionally to initiatives at medical centers.
Discussing the implications: dynamics influencing transitions in genetic healthcare
Development of truly transformative genetic services requires extensive exploration, preparation, implementation, and evaluation (e.g., considering education, infrastructure, finances), which are generally long-term processes. The timely and careful planning of these processes are therefore of utmost importance to drive transitions that improve genetic healthcare (Rigter et al. 2014). The regime descriptions of Estonia, Finland, and the Netherlands are based on discriminators in culture, structure, and practice that provides routes to the expected or required response to dynamics in relevant “niches.” These dynamics could be technology-driven, considering the quickly expanding genetic toolkit, but also public perceptions, values, and needs in (local) regimes or landscapes may change after a disruptive event. Perhaps, the public health crisis caused by the SARS-Cov2 pandemic and its potential implications on, e.g., public trust and views on solidarity and autonomy in addition to lessons from application of genomic sequencing technology in this context, could instigate disruption to cause transformation in healthcare. If true, this will likely also affect clinical genetic service provision.
Technological advancements have increased the demand for high throughput sequencing techniques and genetic testing in general for more and more patients. This has raised discussions about the sustainability of the existing clinical genetic framework in many countries. Prioritizing applications and subsequently allocating budgets to the most (cost)effective tests will require evaluation of many different techniques, interventions, and outcomes. For many reasons outside the scope of this paper, this evaluation will be challenging (Love-Koh et al. 2018; Vrijenhoek et al. 2018).
Furthermore, genetic insights have increased the demand to widen the application of genetic testing beyond diagnosing rare genetic diseases. This may possibly introduce broader implementation of novel service delivery models, e.g., “mainstreaming” to non-genetic medical specialists, and pharmacogenetic support for treatment and genetic testing in the context of disease prevention (Rigter et al. 2014). The extent to which this happens—and how—strongly depends on the local culture, structure, and practice: all influencing the acceptability of certain applications. Of the three countries we have presented here, Estonia, with its modern and tradition-free healthcare, is likely best positioned to widely implement medical genomics in healthcare, at least in the short term. Indeed, the first trials to screen biobank participants and disclose results from testing for familial hypercholesterolemia and breast and ovarian cancer risk are already ongoing (Alver et al. 2019; Leitsalu et al. 2020).
Moreover, the role and position of biobanks in genetic research (and resulting care) have and will significantly influence the genetics regime, including applications for rare diseases. Depending mainly on organizational characteristics and general acceptance of use of genetic data, most likely, there will be an increasing application of genetic analysis for healthy individuals which could be appropriate and feasible for prevention-based healthcare. Uptake of these applications is dependent on the existence of national biobank initiatives and legislative prerequisites, public perceptions of genetic data and its associated aspects of access, identity and uncertainty, and the future position of genetics in the healthcare system, and in society as a whole.
In terms of organization of clinical genetic services, genetic counselors/nurses are still deemed best equipped to address the increasing need for counseling, such as risk communication to non-symptomatic high-risk individuals or by facilitating cascade screening. However, the state of genetic counseling as a profession, or the necessary training in different countries across Europe, varies to a great degree, potentially already leading to suboptimal care in some regions (Abacan et al. 2019). In order to provide good genetic healthcare, the clinical genetic workforce needs to be expanded and optimized in order to meet the current demands, both in terms of quality and quantity. Moreover, there is a considerable lack of applicable genetic knowledge beyond clinical genetics, especially when genetic testing is increasingly being applied outside the traditional clinical genetic regimes, as expected. In addition, new genetic tools and developments require retraining of existing genetic counselors, and possibly the creation of new medical professions specialized in various advanced medical genetic techniques.
Whereas population screening programs currently are generally the responsibility of public health institutes or other national agencies, the expertise and experience within clinical genetics can be instrumental in the development of current and future pre-symptomatic screening strategies. This was particularly apparent in the implementation of non-invasive prenatal testing (NIPT), where—at least in the Netherlands—clinical geneticists took a central role in the development of counseling, training, and testing strategies (van der Meij et al. 2019; van Schendel et al. 2017). A similar attitude could be envisioned for genetic testing to enable informed decision-making in family planning. Especially, rare disease patient organizations seem to be increasingly requesting comprehensive non-invasive prenatal diagnostics, preimplantation genetic diagnostics, and/or preconception carrier screening programs (Bell et al. 2011; Geraedts et al. 2001; Wright and Burton 2008).
Conclusion
Dynamics in niches are influencing the local culture, structure, and practice of genetic services. More widespread application of genetics beyond traditional clinical genetics has the potential to transform genetic healthcare. However, major obstacles including lack of clinical evidence for testing for polygenic traits, ethical, legal, and social challenges and the need for development of innovative reimbursement models remain.
By effectively adapting to dynamics in both the landscape and emerging niches locally, but simultaneously attuning developments and sharing experience across countries in Europe, we believe that optimization of genetic healthcare can ultimately be achieved.
The clinical genetic regime should sustainably use their expertise and experience in the context of diagnostics, counseling, and cascade screening for patients and families with rare diseases. Simultaneously, this knowledge could be translated into novel strategies towards service provision and educating other healthcare professionals. Moreover, continuous education and training of current genetic professionals is needed in order to keep up with (technological) developments in the field. Of crucial importance will be data sharing and breaking down the silo mentality, not only within countries but also between nations.
References
Aartsma-Rus A, Fokkema I, Verschuuren J, Ginjaar I, van Deutekom J, van Ommen G-J, den Dunnen JT (2009) Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat 30(3):293–299. https://doi.org/10.1002/humu.20918
Abacan M, Alsubaie L, Barlow-Stewart K, Caanen B, Cordier C, Courtney E, Davoine E, Edwards J, Elackatt NJ, Gardiner K, Guan Y, Huang L-H, Malmgren CI, Kejriwal S, Kim HJ, Lambert D, Lantigua-Cruz PA, Lee JMH, Lodahl M, Wicklund C (2019) The global state of the genetic counseling profession. Eur J Hum Genet 27(2):183–197. https://doi.org/10.1038/s41431-018-0252-x
Ahola-Launonen J (2016) Social responsibility and healthcare in Finland. Camb Q Healthc Ethics. https://doi.org/10.1017/s0963180116000098
Alver M, Palover M, Saar A, Läll K, Zekavat SM, Tõnisson N, Leitsalu L, Reigo A, Nikopensius T, Ainla T, Kals M, Mägi R, Gabriel SB, Eha J, Lander ES, Irs A, Philippakis A, Marandi T, Natarajan P, Esko T (2019) Recall by genotype and cascade screening for familial hypercholesterolemia in a population-based biobank from Estonia. Genet Med 21(5):1173–1180. https://doi.org/10.1038/s41436-018-0311-2
Amsterdam UMC, Locatie VUmc - Mijn DNAmedicatiepas. Website accessed February 2, 2021, from https://www.vumc.nl/zorg/expertisecentra-en-specialismen/apotheek-en-klinische-farmacologie/mijn-dnamedicatiepas.htm
Battista RN, Blancquaert I, Laberge A-M, van Schendel N, Leduc N (2012) Genetics in health care: an overview of current and emerging models. Pub Health Genom 15(1):34–45. https://doi.org/10.1159/000328846
Bell CJ, Dinwiddie DL, Miller NA, Hateley SL, Ganusova EE, Mudge J, Langley RJ, Zhang L, Lee CC, Schilkey FD, Sheth V, Woodward JE, Peckham HE, Schroth GP, Kim RW, Kingsmore SF (2011) Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci Transl Med 3(65):65ra4. https://doi.org/10.1126/scitranslmed.3001756
Baltictimes.com (2020). Newspaper article: Estonian Health Insurance Fund to start covering cancer screening for uninsured persons. The Baltic Times. Accessed Februari 10, 2021 from: https://www.baltictimes.com/estonian_health_insurance_fund_to_start_covering_cancer_screening_for_uninsured_persons/
Boomsma DI, Wijmenga C, Slagboom EP, Swertz MA, Karssen LC, Abdellaoui A, Ye K, Guryev V, Vermaat M, van Dijk F, Francioli LC, Hottenga JJ, Laros JFJ, Li Q, Li Y, Cao H, Chen R, Du Y, Li N, van Duijn CM (2014) The genome of the Netherlands: design, and project goals. Eur J Hum Genet 22(2):221–227. https://doi.org/10.1038/ejhg.2013.118
Borodulin K, Tolonen H, Jousilahti P, Jula A, Juolevi A, Koskinen S, Kuulasmaa K, Laatikainen T, Männistö S, Peltonen M, Perola M, Puska P, Salomaa V, Sundvall J, Virtanen SM, Vartiainen E (2018) Cohort Profile: The National FINRISK Study. Int J Epidemiol 47(3):696–696i. https://doi.org/10.1093/ije/dyx239
Borry P, van Hellemondt RE, Sprumont D, Jales CFD, Rial-Sebbag E, Spranger TM, Curren L, Kaye J, Nys H, Howard H (2012) Legislation on direct-to-consumer genetic testing in seven European countries. Eur J Hum Genet 20(7):715–721. https://doi.org/10.1038/ejhg.2011.278
Ćwiklicki M, Schiavone F, Klich J, Pilch K (2020) Antecedents of use of e-health services in Central Eastern Europe: a qualitative comparative analysis. BMC Health Serv Res 20(1). https://doi.org/10.1186/s12913-020-5034-9
de Wert G, Dondorp W, Clarke A, Dequeker EMC, Cordier C, Deans Z, van El CG, Fellmann F, Hastings R, Hentze S, Howard H, Macek M, Mendes A, Patch C, Rial-Sebbag E, Stefansdottir V, Cornel MC, Forzano F, Genetics, O. behalf of the E. S. of H (2020) Opportunistic genomic screening. Recommendations of the European Society of Human Genetics. Eur J Hum Genet. https://doi.org/10.1038/s41431-020-00758-w
e-Health Records — e-Estonia (e-estonia.com). Website accessed January 16, 2021, from https://e-estonia.com/solutions/healthcare/e-health-record/
Eloranta K, Auvinen A (2015) Population attitudes towards research use of health care registries: a population-based survey in Finland. BMC Med Ethics 16(1):48. https://doi.org/10.1186/s12910-015-0040-x
The Finnish Disease Database: FinDis.org. Accessed on February 9, 2021, from http://www.findis.org/index.php
Private health care - Sosiaali- ja terveysministeriö (stm.fi). Website accessed January 25, 2021, from https://stm.fi/en/private-health-care
Finngen research project. Website accessed January 25, 2021, from https://www.finngen.fi/en
Johansen F, Loorbach D, Stoopendaal (2018) Exploring a transition in Dutch healthcare. J Health Organ Manag 32(7):875–890. https://doi.org/10.1108/JHOM-07-2018-0185
Rotmans J, Kemp R, van Asselt M (2001) More evolution than revolution: transition management in public policy. Foresight 3(1):15-31. https://doi.org/10.1108/14636680110803003
Geels FW, Schot J (2010) The dynamics of transitions: a socio-technical perspective. In: Grin, Rotmans, Schot (eds) Transitions to Sustainable Development: New Directions in the Study of Long Term Transformative Change. Routledge, New York, pp 11–93
Geraedts JPM, Harper J, Braude P, Sermon K, Veiga A, Gianaroli L, Agan N, Munné S, Gitlin S, Blenow E, de Boer K, Hussey N, Kanavakis E, Lee S-H, Viville S, Krey L, Ray P, Emiliani S, Hsien Liu Y, Vermeulen S (2001) Preimplantation genetic diagnosis (PGD), a collaborative activity of clinical genetic departments and IVF centres. Prenat Diagn 21(12):1086–1092. https://doi.org/10.1002/pd.249
Health Council of the Netherlands (1989). Advisory report Heredity: Science and Society; on the possibilities and limits of genetic testing and gene therapy. Health Council of the Netherlands, The Hague Publication No 89/31. Available from: https://www.healthcouncil.nl/documents/advisory-reports/1989/12/29/heredity-science-and-society-possibilities-limits-genetic-testing-gene-therapy Accessed January 25, 2021
Gilissen C, Hehir-Kwa JY, Thung DT, van de Vorst M, van Bon BWM, Willemsen MH, Kwint M, Janssen IM, Hoischen A, Schenck A, Leach R, Klein R, Tearle R, Bo T, Pfundt R, Yntema HG, de Vries BBA, Kleefstra T, Brunner HG, Veltman JA (2014) Genome sequencing identifies major causes of severe intellectual disability. Nature 511(7509):344–347. https://doi.org/10.1038/nature13394
Haghi M, Thurow K, Stoll R (2017) Wearable devices in medical internet of things: scientific research and commercially available devices. Healthc Inform Res 23(1):4–15. https://doi.org/10.4258/hir.2017.23.1.4
Hamet P, Tremblay J (2017) Artificial intelligence in medicine. Metabolism 69:S36–S40. https://doi.org/10.1016/j.metabol.2017.01.011
Harakalova M, van Harssel JJT, Terhal PA, van Lieshout S, Duran K, Renkens I, Amor DJ, Wilson LC, Kirk EP, Turner CLS, Shears D, Garcia-Minaur S, Lees MM, Ross A, Venselaar H, Vriend G, Takanari H, Rook MB, van der Heyden MAG, Cuppen E (2012) Dominant missense mutations in ABCC9 cause Cantú syndrome. Nat Genet 44(7):793–796. https://doi.org/10.1038/ng.2324
Hardy, G. H. (1908). Mendelian proportions in a mixed population. In Science. https://doi.org/10.1126/science.28.706.49
Healthcare in Finland (2013). Brochures of the Ministry of Social Affairs and Health. Accessed on Februari 10, 2021 from: http://www.urn.fi/URN:ISBN:978-952-00-3395-8
Hofman A, Breteler MMB, van Duijn CM, Krestin GP, Pols HA, Stricker BHC, Tiemeier H, Uitterlinden AG, Vingerling JR, Witteman JCM (2007) The Rotterdam Study: objectives and design update. Eur J Epidemiol 22(11):819–829. https://doi.org/10.1007/s10654-007-9199-x
Hoischen A, van Bon BWM, Gilissen C, Arts P, van Lier B, Steehouwer M, de Vries P, de Reuver R, Wieskamp N, Mortier G, Devriendt K, Amorim MZ, Revencu N, Kidd A, Barbosa M, Turner A, Smith J, Oley C, Henderson A, Veltman JA (2010) De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet 42(6):483–485. https://doi.org/10.1038/ng.581
Holtkamp KCA, Vos EM, Rigter T, Lakeman P, Henneman L, Cornel MC (2017) Stakeholder perspectives on the implementation of genetic carrier screening in a changing landscape. BMC Health Serv Res 17(1):146. https://doi.org/10.1186/s12913-017-2083-9
Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, Ye S, Webb TR, Rutter MK, Tzoulaki I, Patel RS, Loos RJF, Keavney B, Hemingway H, Thompson J, Samani NJ (2018) Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol 72(16):1883–1893. https://doi.org/10.1016/j.jacc.2018.07.079
Isidor B, Julia S, Saugier-Veber P, Weil-Dubuc P-L, Bézieau S, Bieth E, Bonnefont J-P, Munnich A, Bourdeaut F, Bourgain C, Chassaing N, Corradini N, Haye D, Plaisancie J, Dupin-Deguine D, Calvas P, Mignot C, Cogné B, Manouvrier S, Vincent M (2019) Searching for secondary findings: considering actionability and preserving the right not to know. Eur J Hum Genet 27(10):1481–1484. https://doi.org/10.1038/s41431-019-0438-x
Jambroes M, Essink-Bot M-L, Plochg T, Zaadstra B, Stronks K (2013) De Nederlandse publieke gezondheidszorg: 10 kerntaken en een nieuwe definitie. Ned Tijdschr Geneeskd 157:A6195
Keis A (2016) Biobanking in Estonia. J Law Med Ethics. https://doi.org/10.1177/1073110516644186
Kerry VB, Rosa W, Beck D-M, Shaw HK, Morin KH, Breakey S, Evans LA, Dossey BM, Oerther S, Manjrekar P, McKinnon TH, Fitzpatrick JJ, Squires AP, Abboud S, Ojemeni MT, Meleis AI (2017) Transforming our world: the 2030 agenda for sustainable development. In: Rosa W (ed) A New Era in Global Health - Nursing and the United Nations 2030 Agenda for Sustainable Development, 1st edn. Springer Publishing Company, pp 529–567. https://doi.org/10.1891/9780826190123
Keskimaki I, Tynkkynen LK, Reissell E, Koivusalo M, Syrja V, Vuorenkoski L, Rechel B, Karanikolos M (2019) Finland: health system review. Health Syst Transit 21(2):1–166
Kroneman, M., Boerma, W., van den Berg, M., Groenewegen, P., de Jong, J., & van Ginneken, E. (2016). Netherlands: health system review. In Health systems in transition, 18(2):1-240
Lai T, Habicht T, Kahur K, Reinap M, Kiivet R, van Ginneken E (2013) Estonia: health system review. Health Syst Transit 16(6):1–196
Leitsalu L, Alavere H, Jacquemont S, Kolk A, Maillard AM, Reigo A, Nõukas M, Reymond A, Männik K, Ng PC, Metspalu A (2016) Reporting incidental findings of genomic disorder-associated copy number variants to unselected biobank participants. Personal Med 13(4):303–314. https://doi.org/10.2217/pme-2016-0009
Leitsalu L, Alavere H, Tammesoo M-L, Leego E, Metspalu A (2015) Linking a population biobank with national health registries—the Estonian experience. J Personal Med 5(2). https://doi.org/10.3390/jpm5020096
Leitsalu L, Haller T, Esko T, Tammesoo M-L, Alavere H, Snieder H, Perola M, Ng PC, Mägi R, Milani L, Fischer K, Metspalu A (2014) Cohort Profile: Estonian Biobank of the Estonian Genome Center, University of Tartu. Int J Epidemiol 44(4):1137–1147. https://doi.org/10.1093/ije/dyt268
Leitsalu, L., Palover, M., Sikka, T. T., Reigo, A., Kals, M., Parn, K., Nikopensius, T., Esko, T., Metspalu, A., Padrik, P., & Tonisson, N. (2020). Genotype-first approach to the detection of hereditary breast and ovarian cancer risk, and effects of risk disclosure to biobank participants. MedRxiv, 2020.06.29.20139691. https://doi.org/10.1101/2020.06.29.20139691
Loorbach, D., Frantzeskaki, N., & Avelino, F. (2017). Sustainability transitions research: transforming science and practice for societal change. In: Gadgil TP, Tomich A (eds) Annual Review Of Environment And Resources, Annual Reviews, vol 42, pp 599–626. https://doi.org/10.1146/annurev-environ-102014-021340
Love-Koh J, Peel A, Rejon-Parrilla JC, Ennis K, Lovett R, Manca A, Chalkidou A, Wood H, Taylor M (2018) The future of precision medicine: potential impacts for health technology assessment. PharmacoEconomics 36(12):1439–1451. https://doi.org/10.1007/s40273-018-0686-6
Lynch SA, Borg I (2016) Wide disparity of clinical genetics services and EU rare disease research funding across Europe. J Commun Genet 7(2):119–126. https://doi.org/10.1007/s12687-015-0256-y
Maarse H, Jeurissen P, Ruwaard D (2016) Results of the market-oriented reform in the Netherlands: a review. Health Econ Policy Law 2:161–178 https://heinonline.org/HOL/P?h=hein.journals/hecpol11&i=170
Marjonen, H., Marttila, M., & Paajanen, T. (2020). A method to report polygenic risk score results for health care use – P5 Study. Submitted.
Martin GP, Currie G, Finn R (2009) Reconfiguring or reproducing intra-professional boundaries? Specialist expertise, generalist knowledge and the “modernization” of the medical workforce. Soc Sci Med 68(7):1191–1198. https://doi.org/10.1016/j.socscimed.2009.01.006
Matthijs G, Souche E, Alders M, Corveleyn A, Eck S, Feenstra I, Race V, Sistermans E, Sturm M, Weiss M, Yntema H, Bakker E, Scheffer H, Bauer P (2016) Guidelines for diagnostic next-generation sequencing. Eur J Hum Genet 24(1):2–5. https://doi.org/10.1038/ejhg.2015.226
Mendel G (1941) Versuche über Pflanzen-Hybriden. Der Zuchter 13:221–268. https://doi.org/10.1007/BF01804628
Mikselaar RV, Zordania R, Viikmaa M, Kudrjavtseva G (1998) Neonatal screening for congenital hypothyroidism in Estonia. Pediatr Endocrinol Rev 5(1):20–21. https://doi.org/10.1136/jms.5.1.20
Nelis, A. (1999). Managing genetic testing: the relative powerlessness of actors in stable practices. In New Genetics and Society. https://doi.org/10.1080/14636779908656895
Niermeijer MF (2011) Geschiedenis van de klinische genetica. Bijblijven 27(9):7–13. https://doi.org/10.1007/s12414-011-0073-0
Norio R, Nevanlinna H, Perheentupa J (1973) Hereditary diseases in Finland; rare flora in rare soul. Ann Clin Res 5(3):109–141
Norio, Reijo. (2003a). Finnish Disease Heritage I: characteristics, causes, background. In Human Genetics. https://doi.org/10.1007/s00439-002-0875-3
Norio, Reijo. (2003b). Finnish Disease Heritage II: ppulation prehistory and genetic roots of Finns. In Human Genetics. https://doi.org/10.1007/s00439-002-0876-2
Optimity Advisors (2018). Health system performance assessment – integrated care assessment (20157303 HSPA) (Vol. 02, Issue June). https://doi.org/10.2875/81031
Pajusalu S, Kahre T, Roomere H, Murumets Ü, Roht L, Simenson K, Reimand T, Õunap K (2018) Large gene panel sequencing in clinical diagnostics—results from 501 consecutive cases. Clin Genet 93(1):78–83. https://doi.org/10.1111/cge.13031
Pohjola P, Hedley V, Bushby K, Kääriäinen H (2016) Challenges raised by cross-border testing of rare diseases in the European union. Eur J Hum Genet 24(11):1547–1552. https://doi.org/10.1038/ejhg.2016.70
Postelnicu, L. (2019). Estonia, the Netherlands & Nordics continue to drive eHealth adoption and use in Europe, study finds - a new report from HIMSS sheds light on the current state of eHealth in Europe. Healthcare IT News. Accessed Januari 25, 2021 from: Estonia, the Netherlands & Nordics continue to drive eHealth adoption and use in Europe, study finds | Healthcare IT News
Radboud UMC (2018). ZonMw grant to promote large scale (re)use of DNA data. https://www.radboudumc.nl/en/news/2018/zonmw-grant-to-promote-large-scale-reuse-of-dna-data
Reinson, K. (2018). New diagnostic methods for early detection of inborn errors of metabolism in Estonia [Universitatis Tartuensis]. https://dspace.ut.ee/bitstream/handle/10062/62653/reinson_karit.pdf?sequence=4&isAllowed=y
Rigter T, Henneman L, Broerse JEW, Shepherd M, Blanco I, Kristoffersson U, Cornel MC (2014) Developing a framework for implementation of genetic services: learning from examples of testing for monogenic forms of common diseases. J Commun Genet 5(4):337–347. https://doi.org/10.1007/s12687-014-0189-x
Rigter T, Jansen ME, Groot J Md, Janssen SWJ, Rodenburg W, Cornel MC (2020) Implementation of pharmacogenetics in primary care: a multi-stakeholder perspective. Front Genet. https://doi.org/10.3389/fgene.2020.00010
Dutch National Institute for Public Health and the Environment (RIVM) (2010) Handreiking gezonde gemeente. Accessed January 25, 2021, from https://www.loketgezondleven.nl/programmas/gezonde-gemeente/over-de-handreiking
Dutch National Institute for Public Health and the Environment (RIVM) (2021) Website Preventie in Volksgezondheidenzorg.info.nl. Accessed January 25, 2021, from https://www.volksgezondheidenzorg.info/verantwoording/preventie-volksgezondheidenzorginfo/wat-preventie#!node-doelgroepen-van-preventie
Rook MB, Bezzina Alshinawi C, Groenewegen WA, van Gelder IC, van Ginneken ACG, Jongsma HJ, Mannens MMAM, Wilde AAM (1999) Human SCN5A gene mutations alter cardiac sodium channel kinetics and are associated with the Brugada syndrome*. Cardiovasc Res 44(3):507–517. https://doi.org/10.1016/S0008-6363(99)00350-8
Ropers H-H, Hamel BCJ (2005) X-linked mental retardation. Nat Rev Genet 6(1):46–57 http://10.0.4.14/nrg1501
Rotmans, J. (2005). Societal Innovation: between dream and reality lies complexity. https://econpapers.repec.org/RePEc:ems:euriar:7293
Rus D, Tolley MT (2015) Design, fabrication and control of soft robots. Nature 521:467. https://doi.org/10.1038/nature14543
Santos HC, Varnum MEW, Grossmann I (2017) Global increases in individualism. Psychol Sci 28(9):1228–1239. https://doi.org/10.1177/0956797617700622
Scholtens S, Smidt N, Swertz MA, Bakker SJL, Dotinga A, Vonk JM, van Dijk F, van Zon SKR, Wijmenga C, Wolffenbuttel BHR, Stolk RP (2014) Cohort profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol 44(4):1172–1180. https://doi.org/10.1093/ije/dyu229
Sedaghati-Khayat B, Boer CG, Broer L, Verkerk A, Zeggini E, Consortium GO, van Rooij JG, Uitterlinden AG, van Meurs JB (2020) Polygenic risk score and its potential to improve diagnostic ability in knee and hip osteoarthritis. Osteoarthr Cartil 28:S24. https://doi.org/10.1016/j.joca.2020.02.038
Sheiman I, Shishkin S, Shevsky V (2018) The evolving Semashko model of primary health care: the case of the Russian Federation. Risk Manag Healthc Policy 11:209–220. https://doi.org/10.2147/RMHP.S168399
Smeets DFCM, Hamel BCJ, Nelen MR, Smeets HJM, Bollen JHM, Smits APT, Ropers H-H, van Oost BA (1992) Prader–Willi syndrome and Angelman syndrome in cousins from a family with a translocation between chromosomes 6 and 15. N Engl J Med 326(12):807–811. https://doi.org/10.1056/NEJM199203193261206
Soini S (2016) Biobanks as a central part of the Finnish growth and genomic strategies: how to balance privacy in an innovation ecosystem? J Law Med Ethics 44(1):24–34. https://doi.org/10.1177/1073110516644187
Stark Z, Dolman L, Manolio TA, Ozenberger B, Hill SL, Caulfied MJ, Levy Y, Glazer D, Wilson J, Lawler M, Boughtwood T, Braithwaite J, Goodhand P, Birney E, North KN (2019) Integrating genomics into healthcare: a global responsibility. Am J Hum Genet 104(1):13–20. https://doi.org/10.1016/j.ajhg.2018.11.014
Sun, Y., Ruivenkamp, C. A. L., Hoffer, M. J. V, Vrijenhoek, T., Kriek, M., van Asperen, C. J., den Dunnen, J. T., & Santen, G. W. E. (2015). Next-generation diagnostics: gene panel, exome, or whole genome? Hum Mutat, 36(6), 648–655. https://doi.org/10.1002/humu.22783
Meditsiiniuudised (2020) Tervise infosüsteemi hakatakse kaasajastama. Meditsiini-Uudised. Accessed on January 25, 2021 at https://www.mu.ee/uudised/2020/06/08/tervise-infosusteemi-hakatakse-kaasajastama
Tessadori F, Roessler HI, Savelberg SMC, Chocron S, Kamel SM, Duran KJ, van Haelst MM, van Haaften G, Bakkers J (2018) Effective CRISPR/Cas9-based nucleotide editing in zebrafish to model human genetic cardiovascular disorders. Dis Model Mech 11(10). https://doi.org/10.1242/dmm.035469
Tiik M, Ross P (2010) Patient opportunities in the Estonian electronic health record system. Studi Health Technol Inform 156:171–177. https://doi.org/10.3233/978-1-60750-565-5-171
Tjio JH, Levan A (1956) The chromosome number of man. Hereditas 42:1–6. https://doi.org/10.1111/j.1601-5223.1956.tb03010.x
Unim B, Pitini E, Lagerberg T, Adamo G, De Vito C, Marzuillo C, Villari P (2019) Current genetic service delivery models for the provision of genetic testing in Europe: a systematic review of the literature. Front Genet 10:552. https://doi.org/10.3389/fgene.2019.00552
van der Meij KRM, Sistermans EA, Macville MVE, Stevens SJC, Bax CJ, Bekker MN, Bilardo CM, Boon EMJ, Boter M, Diderich KEM, de Die-Smulders CEM, Duin LK, Faas BHW, Feenstra I, Haak MC, Hoffer MJV, den Hollander NS, Hollink IHIM, Jehee FS, Weiss MM (2019) TRIDENT-2: national implementation of genome-wide non-invasive prenatal testing as a first-tier screening test in the Netherlands. Am J Hum Genet. https://doi.org/10.1016/j.ajhg.2019.10.005
van El CG, Cornel MC, Borry P, Hastings RJ, Fellmann F, Hodgson SV, Howard HC, Cambon-Thomsen A, Knoppers BM, Meijers-Heijboer H, Scheffer H, Tranebjaerg L, Dondorp W, de Wert GMWR, on behalf of the Public and Professional Policy Committee (2013) Whole-genome sequencing in health care. Recommendations of the European Society of Human Genetics. Eur J Human Genet 1(Suppl 1):S1–S5. https://doi.org/10.1038/ejhg.2013.46
van Ginneken E, Habicht J, Murauskiene L, Behmane D, Mladovsky P (2012) The Baltic states: building on 20 years of health reforms. BMJ 345:e7348. https://doi.org/10.1136/bmj.e7348
van Raak, R. (2016). Transition policies; connecting system dynamics, governance and instruments in an application to Dutch Healthcare [Erasmus University Rotterdam]. http://hdl.handle.net/1765/80061
van Raak, R., & de Haan, F. J. (2018). Key features of modern health systems. In Toward Sustainable Transitions in Healthcare Systems. https://doi.org/10.4324/9781315232133-3
van Schendel RV, van El CG, Pajkrt E, Henneman L, Cornel MC (2017) Implementing non-invasive prenatal testing for aneuploidy in a national healthcare system: global challenges and national solutions. BMC Health Serv Res 17(1):670. https://doi.org/10.1186/s12913-017-2618-0
Vears DF, Sénécal K, Clarke AJ, Jackson L, Laberge AM, Lovrecic L, Piton A, Van Gassen KLI, Yntema HG, Knoppers BM, Borry P (2018) Points to consider for laboratories reporting results from diagnostic genomic sequencing. Eur J Human Genet 26(1):36–43. https://doi.org/10.1038/s41431-017-0043-9
Veltman J, Cuppen E, Vrijenhoek T (2013) Challenges for implementing next-generation sequencing-based genome diagnostics: it’s also the people, not just the machines. Personal Med 10:473–484. https://doi.org/10.2217/pme.13.41
Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu Y-H, Kuhl DPA, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang F, Eussen BE, van Ommen G-JB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Thomas Caskey C, Nelson DL, Warren ST (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65(5):905–914. https://doi.org/10.1016/0092-8674(91)90397-H
Vissers LELM, van Ravenswaaij CMA, Admiraal R, Hurst JA, de Vries BBA, Janssen IM, van der Vliet WA, Huys EHLPG, de Jong PJ, Hamel BCJ, Schoenmakers EFPM, Brunner HG, Veltman JA, van Kessel AG (2004) Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet 36(9):955–957 http://10.0.4.14/ng1407
Vereniging Klinisch Genetische Laboratoriumdiagnostiek. Website accessed February 9, 2021 from https://www.vkgl.nl/nl/
Vereniging Klinische Genetica Nederland. Website accessed February 9, 2021 from https://www.vkgn.org
Von Koskull H, Salonen R (1997) Genetic services in Finland. Eur J Hum Genet. https://doi.org/10.1159/000484839
Vrijenhoek T, Kraaijeveld K, Elferink M et al (2015) Next-generation sequencing-based genome diagnostics across clinical genetics centers: implementation choices and their effects. Eur J Hum Genet 23:1142–1150. https://doi.org/10.1038/ejhg.2014.279
Vrijenhoek, T., Middelburg, E. M., Monroe, G. R., van Gassen, K. L. I., Geenen, J. W., Hövels, A. M., Knoers, N. V, van Amstel, H. K. P., & Frederix, G. W. J. (2018). Whole-exome sequencing in intellectual disability; cost before and after a diagnosis. Eur J Hum Genet, 26(11), 1566–1571. https://doi.org/10.1038/s41431-018-0203-6
Weinberg, W. (1909). Über Vererbungsgesetze beim Menschen. Zeitschrift Für Induktive Abstammungs- Und Vererbungslehre. https://doi.org/10.1007/BF01975801
Weiss MM, der Zwaag B, Jongbloed JDH, Vogel MJ, Brüggenwirth HT, Lekanne Deprez RH, Mook O, Ruivenkamp CAL, van Slegtenhorst MA, van den Wijngaard A, Waisfisz Q, Nelen MR, van der Stoep N (2013) Best practice guidelines for the use of next-generation sequencing applications in genome diagnostics: a National Collaborative Study of Dutch Genome Diagnostic Laboratories. Hum Mutat 34(10):1313–1321. https://doi.org/10.1002/humu.22368
Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten JW, da Silva Santos LB, Bourne PE, Bouwman J, Brookes AJ, Clark T, Crosas M, Dillo I, Dumon O, Edmunds S, Evelo CT, Finkers R, Mons B (2016) The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. https://doi.org/10.1038/sdata.2016.18
Wittmayer, J. M., Avelino, F., van Steenbergen, F., & Loorbach, D. (2017). Actor roles in transition: insights from sociological perspectives. Environ Innov Soc Transit https://doi.org/10.1016/j.eist.2016.10.003
Wright CF, Burton H (2008) The use of cell-free fetal nucleic acids in maternal blood for non-invasive prenatal diagnosis. Hum Reprod Update 15(1):139–151. https://doi.org/10.1093/humupd/dmn047
Zorginstituut Nederland (2018). End report project: Eindrapportage FAIR Data. Accessed Februari 10, 2021 from: https://www.rijksoverheid.nl/documenten/rapporten/2018/11/15/eindrapportage-fair-data.
Funding
LL and NT have been supported by the European Union through the European Regional Development Fund (project no. 2014-2020.4.01.15-0012), European Union Horizon 2020 (grant no. 810645), and Estonian Research Council (PUT PRG555 and RITA1/01-42-03 grants).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
TR declares to have no conflict of interest.
TV is a consultant to the Copenhagen Institute for Future Studies. HK is a consulting clinical geneticist in BlueprintGenetics laboratory. LL is the founder of CenCo Ltd, a genetic counseling company. NT carries Lab MD responsibilities in two private health care practices in Estonia: one performing non-invasive prenatal testing, and one developing polygenic risk scores for clinical use.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rare Diseases
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vrijenhoek, T., Tonisson, N., Kääriäinen, H. et al. Clinical genetics in transition—a comparison of genetic services in Estonia, Finland, and the Netherlands. J Community Genet 12, 277–290 (2021). https://doi.org/10.1007/s12687-021-00514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-021-00514-7