Abstract
Background
Septin 9 (SEPT9) interacts with multiple oncogenic proteins and is expressed abnormally in several cancers, including hepatocellular carcinoma (HCC). Plasminogen activator inhibitor-1 (PAI-1) promotes tumor formation and progression by modulating the tumor immune microenvironment. CXCR2+ immune cells play a crucial role in HCC formation, progression, and prognosis. The relationship between SEPT9 and PAI-1, and their impact on the HCC immune microenvironment remains unclear.
Methods
Expression levels of SEPT9 and PAI-1 were evaluated by immunohistochemistry (IHC) in HCC and background benign liver (n = 76). Their IHC results were examined for relationships with immune cell markers (CXCR2, CD3, CD15, CD68, and CD163), clinical parameters, and survival outcomes.
Results
Higher grade HCC expressed SEPT9 and PAI-1 more frequently. SEPT9 and PAI-1 expression were associated with each other. PAI-1(+) HCCs had higher intratumoral CXCR2, CD3, CD15, CD68, and CD163 expression compared to PAI-1(−) HCCs, while SEPT9 expression correlated with greater CXCR2+ and CD15+ cell counts in tumor. SEPT9(+) HCC patients had shorter OS, although SEPT9 was not an independent prognostic factor.
Conclusion
SEPT9 is associated with PAI-1, a pro-tumorigenic protein. Both SEPT9 and PAI-1 are linked to advanced HCC grades. SEPT9 and PAI-1 positive HCCs have distinct CXCR2+ immune cell landscapes. Further investigation is needed to elucidate a possible SEPT9/PAI-1 interaction and the clinical utility of SEPT9 IHC in HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Assessment of several cancer-associated signature factors can provide critical and more accurate diagnostic and prognostic information than reliance on a single biomarker. Two such pathologically relevant markers are the cell cycle-related protein Septin9 (SEPT9) and the serine protease inhibitor SERPINE1, also known as plasminogen activator inhibitor-1 (PAI-1). SEPT9 is a scaffold protein that interacts with cytoskeletal proteins and plasma membranes [1, 2] and is involved in various fundamental cellular processes, including cytokinesis, vesicle trafficking, adhesion, and motility [3, 4]. Abnormal levels of SEPT9 mRNA or protein are evident across multiple cancers [5, 6], namely breast [7, 8], ovarian [9, 10], colorectal [11], and hepatocellular carcinoma (HCC) [12]. PAI-1 was initially identified as an inhibitor of urokinase-type plasminogen activator (uPA) [13]. It has been demonstrated that PAI-1 is not merely a fibrinolysis inhibitor but acts as a key driver in various human malignancies [14] by stimulating angiogenesis [15], inducing cell migration [16], and evading apoptosis [17].
Our previous immunohistochemistry (IHC) study illustrated that malignant hepatic lesions are more likely to be SEPT9 positive than benign or precursor hepatic lesions, and SEPT9 expression correlates with higher tumor grade in HCC [12]. These findings suggest a possible role of SEPT9 in HCC formation and progression. However, the mechanism by which SEPT9 promotes HCC is not fully understood. Similarly, PAI-1 and its substrate uPA are significantly increased in HCC compared to the adjacent uninvolved liver, and their expression correlates with advanced tumor grade, invasion, and metastasis [18]. Indeed, HCC patients with higher PAI-1 expression have worse overall survival (OS) and recurrence-free survival (RFS) [19]. Notably, recent studies also highlighted PAI-1 as a regulator of the tumor immune microenvironment [20,21,22]. This is particularly relevant since multiple immune cell lineages, including neutrophils and macrophages, express CXCR2 [23, 24]. CXCR2 ligands stimulate HCC cell proliferation and migration in vitro [25] and likely do so in the tumor microenvironment since CXCL8, a ligand for CXCR2, is upregulated as HCC develops in cirrhotic liver [26]. In murine models, small molecule inhibitors of CXCR2 or CXCR2 knockout diminished tumor growth and progression [27, 28]. Indeed, human studies confirmed that CXCR2+ cells are important contributors to HCC progression and bear prognostic significance [29, 30]. Collectively, these findings suggest a possible link between PAI-1 and CXCR2+ immune cells in the HCC immune microenvironment.
The study is the first to evaluate the relationship between SEPT9, PAI-1, and the tumor immune microenvironment in HCC. To this end, SEPT9, PAI-1, and immune marker expression in HCC and in the background benign liver were assessed by IHC. Special attention was paid to CXCR2+ immune cells in the HCC microenvironment. The potential prognostic implications of SEPT9 and PAI-1 in HCC were also evaluated.
2 Methods
This study was approved by the Institutional Review Board (IRB) at Albany Medical College (Albany, NY, USA) (protocol #5718, approval date: 06/18/2020) The IRB granted a waiver of informed patient consent, determining that the study qualified as secondary research for which informed patient consent is not required. All methods were carried out in accordance with relevant guidelines and regulations. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
2.1 Study population
Archived partial hepatectomies (n = 76; 2003–2019) for HCC were retrieved from Albany Medical Center. Neither explants nor biopsies were included in our cohort. Fibrolamellar type HCC and combined hepatocellular-cholangiocarcinoma were excluded.
Electronic medical records and tumor registries were reviewed to obtain demographic features (age, sex), tumor characteristics (tumor size, AFP level at diagnosis, presence of multifocal hepatic lesions, T stage), HCC risk factors (family history of HCC, hepatitis B, hepatitis C, cirrhosis in background liver, past and current alcohol use, smoking history), metabolic dysfunction-associated liver disease (MASLD) risk factors (hypertension, dyslipidemia, diabetes, obesity), other medical conditions, and survival outcomes.
2.2 Histologic review
Archived hematoxylin & eosin (H&E)-stained slides were reviewed. Tumor grades were assigned per the 2019 WHO grading system [31]. For samples containing areas with different grades, the highest grade was assigned. For SEPT9 IHC, representative tissue blocks showing tumor-benign liver interface were selected for each case. For tissue microarray (TMA) construction, slides showing tumor and/or benign liver tissue were selected and the areas to be sampled were circled. Antibody clones, titers, and detection kits used in this study are summarized in Table 1.
2.3 Tissue microarray construction
TMAs (core diameter 1 mm, Galileo TMA CK3600 Computer Driven; Integrated Systems Engineering, Brugherio, Italy) were constructed from corresponding formalin-fixed paraffin-embedded (FFPE) tissue blocks. From each HCC tissue and benign liver, up to 10 and 5 cores were randomly selected, respectively. The constructed TMAs were used for PAI-1, CXCR2, CD3, CD15, CD68, and CD163 IHC.
2.4 SEPT9 immunohistochemical assays
5-micron thick whole sections representative of the tumor-benign liver interface were prepared for SEPT9 immunostaining. Native interlobular bile ducts served as positive internal controls. SEPT9 staining results were dichotomized into positive (≥ 5% of lesion staining) and negative (< 5% of lesion staining). In our previous study, SEPT9 staining was considered positive when > 5% of tumor cells exhibited distinct membranous/cytoplasmic staining with membranous accentuation compared with the benign liver [12]. In this study, any staining within the tumor, including weak cytoplasmic granular pattern, was considered positive if it spanned ≥ 5% of the tissue area.
2.5 PAI-1 immunohistochemical assays
5-micron thick TMA sections were prepared for PAI-1 immunostaining. Fibroblasts within desmoplasia were used as positive controls [32]. Any granular cytoplasmic and/or membranous staining within hepatocytes or tumor cells was considered positive.
2.6 Immune marker immunohistochemical assays
CXCR2, CD15, CD3, CD68, and CD163 IHC were performed using 5-micron thick sections from the TMAs. CXCR2 and CD15 staining were localized in the nucleus and/or cytoplasm of inflammatory cells [33]. The number of CXCR2+ and CD15+ inflammatory cells was counted at a hotspot in a ×400 high power field. CD3+, CD68+, and CD163+ inflammatory cells were considered uncountable since numerous cells were stained by the antibodies. Therefore, their staining extents, instead of cell counts, were assessed and scored as + 1, + 2, and + 3. The means of CXCR2+ and CD15+ cell counts in HCC and benign liver cores were selected as representative values. For CD3, CD68, and CD163 expression levels, the median staining extents in HCC and benign liver cores were used for the analyses.
2.7 Statistical analysis
Statistical analysis was performed using R version 4.3.3, with a p-value < 0.05 as a threshold for statistical significance. Continuous variables were reported as range (mean, median), and categorical variables were presented as frequencies and proportions (%). Normality and equality of variances for continuous variables were evaluated using Shapiro–Wilk test and Levene’s test. Depending on distribution, either T-test or Wilcoxon rank sum test was applied to identify their relationship with PAI-1 and SEPT9 IHC results. PAI-1/SEPT9 expression with respect to categorical variables were analyzed by Fisher’s exact test and if needed, post-hoc analysis by false discovery rate (FDR) adjusted p-value. Ordinal variables (grade and T stage) were further assessed by Cochran-Armitage trend test.
Survival analysis was conducted with Kaplan–Meier estimation and Cox proportional hazards model. Log-rank test was performed to evaluate Kaplan–Meier estimation. Simple Cox regression was performed for each variable, and those with p < 0.05 were then included in multiple Cox regression to identify independent prognostic factors. The statistical significance of the multiple Cox regression model was tested using the Wald test. Clinical features and selected IHC markers (CXCR2, CD15, SEPT9, and PAI-1) were analyzed as potential prognostic factors. The proportional hazard assumption was evaluated for each predictor following simple Cox regression, and variables that do not meet the assumption were excluded from the analysis.
3 Results
3.1 Study population
Demographics and clinical information of the cohort are outlined in Table 2. The mean age at HCC diagnosis was 65 (range 37–88) years; 48 (63.2%) patients were male. The mean tumor size was 6.4 (range 0.8–20) cm. AFP levels at diagnosis were available in 51 patients and AFP levels exceed 400 ng/ml in 10 (19.2%) of them. 9.9% (7) had more than one hepatic lesion. There were 17 (22.4%) grade 1, 38 (50%) grade 2, and 21 (27.6%) grade 3 cases. The mean follow-up was 61.2 (median: 46, range: 0–210) months.
3.2 SEPT9 and PAI-1 are related to each other and associated with higher tumor grade
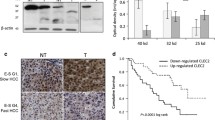
SEPT9 IHC results were available for 68 cases, with 34 (50%) being SEPT9(+) within the tumor. Representative images of SEPT9 IHC are shown in Fig. 1. 22 (29%) cases showed weak cytoplasmic granular staining in neighboring benign liver. SEPT9 staining in the benign region was strongly associated with SEPT9 positivity in HCC (p = 0.02). However, SEPT9 staining in the benign liver was not associated with any of the patient characteristics or immune marker expression (CD3, CD15, CD68, CD163, CXCR2, and PAI-1), thus staining in the benign liver tissue was disregarded. PAI-1 IHC results were available for all 76 cases, with 17 (22%) cases being PAI-1(+) within the tumor (Fig. 2). None of the adjacent benign livers was PAI-1 positive. SEPT9 and PAI-1 staining positivity were associated with each other (p = 0.02).
Table 2 outlines clinical characteristics and their association with SEPT9 and PAI-1. SEPT9 and PAI-1 staining in HCC showed a significant difference according to tumor grade (SEPT9: p = 0.04, PAI-1: p < 0.01). Post-hoc analysis revealed that SEPT9 and PAI-1 expression were more frequently observed in grade 3 and grade 2 HCC patients compared to those with grade 1 (Table 2). Additionally, there was a significant trend indicating that patients with higher tumor grades were more likely to be SEPT9(+) (p = 0.02) and PAI-1(+) (p < 0.01). Other clinical or pathologic features were not significantly associated with SEPT9 and PAI-1 staining.
3.3 SEPT9 and PAI-1 positive HCC have distinct intratumoral CXCR2 + immune cell makeup
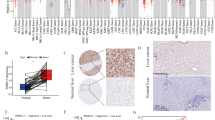
Next, we investigated whether SEPT9 or PAI-1 expression is related to CXCR2+ immune cell makeup in HCC. Specific CXCR2+ cell types, CD15 (neutrophils), CD68 (pan-macrophage), CD163 (M2 macrophage), and CD3 (T lymphocytes) were identified by IHC (See methods). Representative images for CD3, CD68, and CD163 IHC with staining extent scores are shown in Fig. 3. Results are summarized in Table 3. SEPT9(+) HCCs had a higher CXCR2+ (p < 0.01) and CD15+ (p = 0.02) cell count within the tumor. Intratumoral PAI-1 staining showed a positive correlation with CXCR2+ cell count (p = 0.01), CD3 expression (p = 0.04), CD15+ cell count (p < 0.01), CD68 expression (p = 0.03), and CD163 expression (p = 0.03) within the tumor.
3.4 SEPT9 and PAI-1 expression in HCC and benign liver CXCR2+ immune cell makeup
Neither SEPT9 nor PAI-1 expression was significantly associated with CXCR2+ cell counts or specific CXCR2+ cell marker expression (CD3, CD15, CD68, and CD163) in the adjacent benign liver (Table 3, Online Resource 1). However, SEPT9(+) HCC had marginally increased CXCR2+ [p = 0.15, mean in SEPT9(−): 21.8 vs. mean in SEPT9(+): 31.4] and CD15+ [p = 0.08, mean in SEPT9(−): 42.3 vs. mean in SEPT9(+): 56.7] cell count in the uninvolved benign liver compared to SEPT9(−) HCC.
3.5 SEPT9 and PAI-1 influence overall survival in HCC patients only when the other is absent
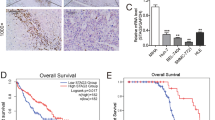
Previous studies suggested that CXCR2+ and CD15+ cells have prognostic value in HCC [29, 30]. SEPT9, PAI-1, CXCR2, and CD15 IHC results were examined for possible associations with oncologic outcomes (Fig. 4, Table 4). Kaplan–Meier analysis revealed that SEPT9(+) HCC patients had shorter OS compared to SEPT9(−) HCC patients (p = 0.01) (Fig. 4a). No significant association was found between PAI-1 staining and OS (p = 0.07) (Fig. 4d). Interestingly, worse OS was associated with SEPT9 or PAI-1 expression only when the other marker was absent (Fig. 4b, c, e, f). Neither SEPT9 nor PAI-1 was related to RFS or 5-year survival rate.
Simple Cox regression for OS identified tumor size (p = 0.03), CD15+ cell counts in the benign liver (p < 0.01), and SEPT9 expression (p = 0.02) as significant prognostic factors. The multiple Cox regression with tumor size, benign liver CD15+ cells, and SEPT9 was performed. Multiple regression model was statistically significant (Wald test, p < 0.01). SEPT9 was not an independent predictor of worse prognosis (p = 0.29), while tumor size [adjusted HR = 1.13, 95% CI (1.03–1.24), p = 0.01] and benign CD15+ cell counts [adjusted HR = 1.02, 95% CI (1.00–1.03), p = 0.02] retained their significance.
4 Discussion
Previous studies demonstrated that SEPT9 interacts with various tumorigenic proteins, such as HIF-1alpha [34], JNK, cyclin D [7], and Rho [35]. In this study, we showed that PAI-1, another pro-tumorigenic protein, is associated with SEPT9 in HCC. Moreover, PAI-1 and SEPT9 protein expression correlated with higher HCC grade, consistent with findings from other cancers [12, 36,37,38].
Growing evidence has suggested that PAI-1 modulates the tumor immune microenvironment, especially in relation to CXCR2+ immune cells. PAI-1 induced PD-L1 expression, and a PAI-1 inhibitor increased the number of CD8+ T cells in the tumor microenvironment while decreasing that of regulatory T cells [21]. Gene expression array analysis demonstrated that PAI-1 is positively correlated with CD163 (M2 macrophage marker) in various cancers [20]. Further, PAI-1 stimulates macrophages to infiltrate into tumors and adopt a pro-tumoral M2 phenotype [20]. PAI-1-uPA dimer has a similar effect on neutrophils [22]. Importantly, CXCL8, a CXCR2 ligand, expression in colon cancer is induced by PAI-1 [39]. Our findings that PAI-1(+) HCCs had higher intratumoral CD3 (T lymphocytes), CD15 (neutrophils), CD68 (pan-macrophage), and CD163 (M2 macrophage) expression, are in line with the previous studies.
On the other hand, the role of SEPT9 in the tumor immune microenvironment is not fully understood, although several studies reported a potential link between SEPT9 and immunological processes [3, 40, 41]. Jiao et al., showed that monocytes incubated with conditioned media from irradiated, SEPT9-overexpressing HeLa cells differentiated into M2 macrophages [42]. Our results showed that SEPT9(+) HCCs have a higher number of intratumoral CXCR2+ and CD15+ cells compared to SEPT9(−) HCC. CXCR2+ and CD15+ cells were highlighted as important mediators of HCC tumorigenesis and progression [29, 30]. Thus, CXCR2+ cells in the tumor microenvironment might serve as a link between SEPT9 and HCC progression.
Interestingly, although patients with SEPT9(+) HCCs tend to also express SEPT9 in neighboring benign liver (p = 0.02), SEPT9 staining in benign regions was not associated with any of the immune markers (CXCR2, CD3, CD15, CD68, and CD163). It is possible that aberrant SEPT9 protein expression initiates from background benign liver tissue in a subset of SEPT9(+) HCCs. Alternatively, SEPT9 expression may be an early event in HCC tumorigenesis, extending to adjacent benign tissue as HCC progresses. Further studies are required to determine the significance of SEPT9 in benign tissue.
It is worth noting that SEPT9(+) and PAI-1(+) HCCs have distinct immune landscapes. Li et al., showed that neutrophils, macrophages, and T lymphocytes are the major subpopulations of CXCR2+ cells in HCC [29]. Intratumoral PAI-1 expression showed a positive correlation with CXCR2+ cell count and specific CXCR2+ cell marker (CD3, CD15, CD68, and CD168) expressions. However, only CXCR2+ and CD15+ cell counts were significantly associated with SEPT9. SEPT9(+) HCC did not exhibit higher CD3, CD68 and CD163 expression levels compared to SEPT9(−) HCC (Table 3). This implies SEPT9 IHC can identify HCCs with distinct intratumoral CXCR2+ immune cell composition, which in turn may harbor clinical significance.
In our HCC cohort, intratumoral SEPT9 expression was associated with shorter OS. Similarly, Stanbery et al., demonstrated that in head and neck cancer, higher SEPT9 IHC intensity correlated with worse oncologic outcomes [43]. However, SEPT9 was not an independent prognostic factor in multiple Cox regression, while CD15+ cell count in benign tissue, which was marginally associated with SEPT9 expression, continued to show significance. Previous studies identified peritumoral CD15+ cell count as an independent predictor for shorter OS and RFS in HCC patients [30], while the exact definition of ‘peritumoral’ was not available. Since our benign liver tissue cores were sampled adjacent to the HCC, the impact of SEPT9 expression on OS might be partly due to higher CD15+ cell count in ‘peritumoral’ benign tissue.
PAI-1 expression is an indicator of worse prognosis in various cancers, including breast [44], colorectal [45], gastric [46], ovarian [47], esophageal cancer [48], and HCC [19]. However, in our cohort, although patients with PAI-1(+) HCC tended to show shorter overall survival (p = 0.07), such an association was not statistically significant. Profound regional differences in HCC risk factors [49, 50] or small cohort size might be responsible for this discrepancy.
Considering that SEPT9 and PAI-1 expression are related, and their common association with CXCR2+ and CD15+ cell count within HCC, it is possible that they interact via modulating the HCC tumor microenvironment. Interestingly, despite their strong correlation, SEPT9 and PAI-1 affect overall survival only when the other is absent (Fig. 4). Their influences on survival outcomes and existence of a possible interaction should be investigated in future studies.
One limitation of our study is that we were unable to differentiate specific isoforms of SEPT9. SEPT9 undergoes complex alternative splicing, with each isoform having distinct effects on tumorigenesis [7, 11]. Additionally, we could not distinguish specific subtypes of CD3+ T cells and CD68+ macrophages, although M2 macrophage was identified by CD163 IHC. Distinct T cell subpopulations, such as CD4+, CD8+, and regulatory T cells, differentially influence HCC development and prognosis [51]. Likewise, M1 macrophages are known to have anti-tumor effects, while anti-inflammatory M2 macrophages promote tumor progression [52]. Kupffer cells, the resident macrophages of the liver, play a crucial role in hepatic fibrosis [53] and non-alcoholic fatty liver disease [54], both of which are well-established risk factors of HCC. Therefore, identifying specific T cell and macrophage subtypes is necessary for the comprehensive understanding of the HCC immune microenvironment. Finally, small cohort size might have led to limited statistical power, especially in subgroup analysis.
To the best of our knowledge, this is the first study to elucidate the relationship between SEPT9, PAI-1, and the HCC immune microenvironment. SEPT9(+) HCCs had a distinct immune microenvironment, with potential clinical implications. Further research is required to validate the existence and mechanism of PAI-1/SEPT9 interaction. Additionally, the potential clinical utility of SEPT9 IHC in HCC should be explored. Of note, small molecule inhibitors of CXCR2 and PAI-1 have been developed and are under clinical investigation [23, 32, 55]. In the future, SEPT9 IHC could provide additional insights regarding these novel therapeutics.
Data availability
The dataset for this study can be obtained from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Dolat L, Hu Q, Spiliotis ET. Septin functions in organ system physiology and pathology. Biol Chem. 2014;395(2):123–41.
Hall PA, Russell SE. The pathobiology of the septin gene family. J Pathol. 2004;204(4):489–505.
Ivanov AI, Le HT, Naydenov NG, Rieder F. Novel functions of the septin cytoskeleton: shaping up tissue inflammation and fibrosis. Am J Pathol. 2021;191(1):40–51.
Chacko AD, Hyland PL, McDade SS, Hamilton PW, Russell SH, Hall PA. SEPT9_v4 expression induces morphological change, increased motility and disturbed polarity. J Pathol. 2005;206(4):458–65.
Scott M, Hyland PL, McGregor G, Hillan KJ, Russell SE, Hall PA. Multimodality expression profiling shows SEPT9 to be overexpressed in a wide range of human tumours. Oncogene. 2005;24(29):4688–700.
Sun J, Zheng MY, Li YW, Zhang SW. Structure and function of Septin 9 and its role in human malignant tumors. World J Gastrointest Oncol. 2020;12(6):619–31.
Gonzalez ME, Makarova O, Peterson EA, Privette LM, Petty EM. Up-regulation of SEPT9_v1 stabilizes c-Jun-N-terminal kinase and contributes to its pro-proliferative activity in mammary epithelial cells. Cell Signal. 2009;21(4):477–87.
Gonzalez ME, Peterson EA, Privette LM, Loffreda-Wren JL, Kalikin LM, Petty EM. High SEPT9_v1 expression in human breast cancer cells is associated with oncogenic phenotypes. Cancer Res. 2007;67(18):8554–64.
Burrows JF, Chanduloy S, McIlhatton MA, Nagar H, Yeates K, Donaghy P, Price J, Godwin AK, Johnston PG, Russell SE. Altered expression of the septin gene, SEPT9, in ovarian neoplasia. J Pathol. 2003;201(4):581–8.
Scott M, McCluggage WG, Hillan KJ, Hall PA, Russell SE. Altered patterns of transcription of the septin gene, SEPT9, in ovarian tumorigenesis. Int J Cancer. 2006;118(5):1325–9.
Toth K, Galamb O, Spisak S, Wichmann B, Sipos F, Valcz G, Leiszter K, Molnar B, Tulassay Z. The influence of methylated septin 9 gene on RNA and protein level in colorectal cancer. Pathol Oncol Res. 2011;17(3):503–9.
Kmeid M, Park YN, Chung T, Pacheco RR, Arslan ME, Lee H. SEPT9 expression in hepatic nodules: an immunohistochemical study of hepatocellular neoplasm and metastasis. Appl Immunohistochem Mol Morphol. 2023;31(5):278–87.
Morrow GB, Mutch NJ. Past, present, and future perspectives of plasminogen activator inhibitor 1 (PAI-1). Semin Thromb Hemost. 2023;49(3):305–13.
Kubala MH, DeClerck YA. The plasminogen activator inhibitor-1 paradox in cancer: a mechanistic understanding. Cancer Metastasis Rev. 2019;38(3):483–92.
Li S, Wei X, He J, Tian X, Yuan S, Sun L. Plasminogen activator inhibitor-1 in cancer research. Biomed Pharmacother. 2018;105:83–94.
Sillen M, Declerck PJ. A narrative review on plasminogen activator inhibitor-1 and its (patho)physiological role: to target or not to target? Int J Mol Sci. 2021;22(5):2721.
Sobel BE, Chen Y, Schneider DJ. The effect of plasminogen activator inhibitor type 1 on apoptosis. Thromb Haemost. 2017;100(12):1037–40.
Bharadwaj AG, Holloway RW, Miller VA, Waisman DM. Plasmin and plasminogen system in the tumor microenvironment: implications for cancer diagnosis, prognosis, and therapy. Cancers (Basel). 2021;13(8):1838.
Jin Y, Liang ZY, Zhou WX, Zhou L. Expression, clinicopathologic and prognostic significance of plasminogen activator inhibitor 1 in hepatocellular carcinoma. Cancer Biomark. 2020;27(3):285–93.
Kubala MH, Punj V, Placencio-Hickok VR, Fang H, Fernandez GE, Sposto R, DeClerck YA. Plasminogen activator inhibitor-1 promotes the recruitment and polarization of macrophages in cancer. Cell Rep. 2018;25(8):2177-2191 e2177.
Ibrahim AA, Fujimura T, Uno T, Terada T, Hirano KI, Hosokawa H, Ohta A, Miyata T, Ando K, Yahata T. Plasminogen activator inhibitor-1 promotes immune evasion in tumors by facilitating the expression of programmed cell death-ligand 1. Front Immunol. 2024;15:1365894.
Uhl B, Mittmann LA, Dominik J, Hennel R, Smiljanov B, Haring F, Smiljanov JB, Braun C, Padovan L, Pick R, et al. uPA-PAI-1 heteromerization promotes breast cancer progression by attracting tumorigenic neutrophils. EMBO Mol Med. 2021;13(6): e13110.
Lazennec G, Rajarathnam K, Richmond A. CXCR2 chemokine receptor - a master regulator in cancer and physiology. Trends Mol Med. 2024;30(1):37–55.
Cheng Y, Ma XL, Wei YQ, Wei XW. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer. 2019;1871(2):289–312.
Song X, Wang Z, Jin Y, Wang Y, Duan W. Loss of miR-532-5p in vitro promotes cell proliferation and metastasis by influencing CXCL2 expression in HCC. Am J Transl Res. 2015;7(11):2254–61.
Nomura T, Morishita A, Jian G, Mimura S, Kato K, Nomura K, Tani J, Miyoshi H, Yoneyama H, Sakamoto T, et al. Expression of angiogenic factors in hepatocarcinogenesis: Identification by antibody arrays. Oncol Rep. 2013;30(5):2476–80.
Saintigny P, Massarelli E, Lin S, Ahn YH, Chen Y, Goswami S, Erez B, O’Reilly MS, Liu D, Lee JJ, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res. 2013;73(2):571–82.
Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122(9):3127–44.
Li L, Xu L, Yan J, Zhen ZJ, Ji Y, Liu CQ, Lau WY, Zheng L, Xu J. CXCR2-CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:129.
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54(5):948–55.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8.
Czekay RP, Higgins CE, Aydin HB, Samarakoon R, Subasi NB, Higgins SP, Lee H, Higgins PJ. SERPINE1: role in cholangiocarcinoma progression and a therapeutic target in the desmoplastic microenvironment. Cells. 2024;13(10):796.
Park E, Subasi NB, Wang X, Kmeid M, Chen A, Tooke-Barry C, Lee H. CXCR2 expression is associated with prostate-specific membrane antigen expression in hepatocellular carcinoma: reappraisal of tumor microenvironment and angiogenesis. Clin Transl Oncol. 2024. https://doi.org/10.1007/s12094-024-03789-7.
Amir S, Wang R, Matzkin H, Simons JW, Mabjeesh NJ. MSF-A interacts with hypoxia-inducible factor-1alpha and augments hypoxia-inducible factor transcriptional activation to affect tumorigenicity and angiogenesis. Cancer Res. 2006;66(2):856–66.
Nagata K, Inagaki M. Cytoskeletal modification of Rho guanine nucleotide exchange factor activity: identification of a Rho guanine nucleotide exchange factor as a binding partner for Sept9b, a mammalian septin. Oncogene. 2005;24(1):65–76.
Mazzoccoli G, Pazienza V, Panza A, Valvano MR, Benegiamo G, Vinciguerra M, Andriulli A, Piepoli A. ARNTL2 and SERPINE1: potential biomarkers for tumor aggressiveness in colorectal cancer. J Cancer Res Clin Oncol. 2012;138(3):501–11.
Castelló R, España F, Vázquez C, Fuster C, Almenar SM, Aznar J, Estellés A. Plasminogen activator inhibitor-1 4G/5G polymorphism in breast cancer patients and its association with tissue PAI-1 levels and tumor severity. Thromb Res. 2006;117(5):487–92.
Gilad R, Meir K, Stein I, German L, Pikarsky E, Mabjeesh NJ. High SEPT9_i1 protein expression is associated with high-grade prostate cancers. PLoS ONE. 2015;10(4): e0124251.
Terada H, Urano T, Konno H. Association of interleukin-8 and plasminogen activator system in the progression of colorectal cancer. Eur Surg Res. 2005;37(3):166–72.
Robertin S, Mostowy S. The history of septin biology and bacterial infection. Cell Microbiol. 2020;22(4): e13173.
Lassen LB, Füchtbauer A, Schmitz A, Sørensen AB, Pedersen FS, Füchtbauer EM. Septin9 is involved in T-cell development and CD8+ T-cell homeostasis. Cell Tissue Res. 2013;352(3):695–705.
Jiao X, Zhang S, Jiao J, Zhang T, Qu W, Muloye GM, Kong B, Zhang Q, Cui B. Promoter methylation of SEPT9 as a potential biomarker for early detection of cervical cancer and its overexpression predicts radioresistance. Clin Epigenetics. 2019;11(1):120.
Stanbery L, D’Silva NJ, Lee JS, Bradford CR, Carey TE, Prince ME, Wolf GT, Worden FP, Cordell KG, Petty EM. High SEPT9_v1 expression is associated with poor clinical outcomes in head and neck squamous cell carcinoma. Transl Oncol. 2010;3(4):239–45.
Duffy MJ, McGowan PM, Harbeck N, Thomssen C, Schmitt M. uPA and PAI-1 as biomarkers in breast cancer: validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014;16(4):428.
Sakakibara T, Hibi K, Koike M, Fujiwara M, Kodera Y, Ito K, Nakao A. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of colorectal cancer. Br J Cancer. 2005;93(7):799–803.
Nekarda H, Schmitt M, Ulm K, Wenninger A, Vogelsang H, Becker K, Roder JD, Fink U, Siewert JR. Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res. 1994;54(11):2900–7.
Chambers SK, Ivins CM, Carcangiu ML. Plasminogen activator inhibitor-1 is an independent poor prognostic factor for survival in advanced stage epithelial ovarian cancer patients. Int J Cancer. 1998;79(5):449–54.
Sakakibara T, Hibi K, Kodera Y, Ito K, Akiyama S, Nakao A. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10(4):1375–8.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma Nat Rev Dis Primers. 2021;7(1):6.
Zheng X, Jin W, Wang S, Ding H. Progression on the roles and mechanisms of tumor-infiltrating T lymphocytes in patients with hepatocellular carcinoma. Front Immunol. 2021;12: 729705.
Huang J, Wu Q, Geller DA, Yan Y. Macrophage metabolism, phenotype, function, and therapy in hepatocellular carcinoma (HCC). J Transl Med. 2023;21(1):815.
Cheng D, Chai J, Wang H, Fu L, Peng S, Ni X. Hepatic macrophages: key players in the development and progression of liver fibrosis. Liver Int. 2021;41(10):2279–94.
Park SJ, Garcia Diaz J, Um E, Hahn YS. Major roles of kupffer cells and macrophages in NAFLD development. Front Endocrinol (Lausanne). 2023;14:1150118.
Nam DE, Seong HC, Hahn YS. Plasminogen activator inhibitor-1 and oncogenesis in the liver disease. J Cell Signal. 2021;2(3):221–7.
Acknowledgements
We thank Ms. Rebecca Pirri for her support for clinical research laboratory operation in Albany Medical Center.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
H.L. contributed to the study conception and design. Anti PAI-1 antibody was provided by P.J.H. Material preparation, data collection and analysis were performed by E.P., X.W., N.B.S., M.K., and H.L. The first draft of the manuscript was written by E.P. and all authors commented on previous versions of the manuscript. A.C. and P.J.H. revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, E., Wang, X., Subasi, N.B. et al. SEPT9 and PAI-1 are immunohistochemical biomarkers of the hepatocellular carcinoma immune microenvironment. Discov Onc 16, 483 (2025). https://doi.org/10.1007/s12672-025-02252-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-025-02252-5