Abstract
Background
This study seeks to investigate the relationship between plasma metabolites or proteins and the risk of ovarian cancer through Mendelian randomization analysis and construct, while also developing a predictive model for resistance to chemotherapy.
Methodology/Principal findings
Appropriate SNPs from GWAS data were selected as instrumental variables. Multiple methods, such as IVW, MR-Egger regression, and WME, were employed to investigate the causal relationship. A predictive model was established utilizing binary logistic regression based on the identified plasma protein genes. Four plasma metabolites and four plasma proteins were recognized as risk factors for ovarian cancer, whereas four plasma proteins were identified as protective factors. A predictive model for chemotherapy resistance was formulated with an AUC of 0.844 (p = 0.002).
Conclusions
Plasma metabolites and proteins may affect the risk of ovarian cancer and its resistance to chemotherapy. This study presents potential predictive factors and the underlying mechanisms influencing the onset, progression, and resistance of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Ovarian cancer is recognized as a common malignancy in the field of gynecology, consistently holding the highest mortality rate among gynecological tumors worldwide [1]. In 2020, it was estimated that over 310,000 new cases of ovarian cancer emerged globally, resulting in roughly 207,200 fatalities [2]. The insidious development of ovarian cancer often leads to patients being asymptomatic during the early stages, and the absence of effective screening methods results in the majority of diagnoses occurring at advanced stages (III or IV) [3]. Despite receiving standard treatment, a considerable proportion of patients face recurrence within 5 years, with the time between recurrences decreasing. Ultimately, resistance to platinum-based drugs may contribute to patient mortality, culminating in a mere 25% 5-year survival rate for those with advanced ovarian cancer [4, 5]. Recently, various researchers have focused on identifying biomarkers for early ovarian cancer screening and predicting drug resistance. CA-125 has been recognized as a potential biomarker for ovarian cancer screening; however, its sensitivity and specificity are constrained, accompanied by a notably high false-positive rate [6,7,8]. As research has advanced, the combined assessment of CA-125 and HE4 has surfaced as a more reliable predictor of ovarian cancer [9]. Nevertheless, the accuracy remains limited, highlighting an urgent necessity for novel biomarkers to forecast the occurrence, progression, and drug resistance associated with ovarian cancer.
Multiple studies have shown a strong correlation between plasma metabolites, plasma proteins, and the onset, progression, and drug resistance of ovarian cancer [10,11,12,13]. Nonetheless, these studies often encounter limitations, including small sample sizes and vulnerability to unmeasured confounding variables, reverse causation, and other influences, making them more prone to bias. Furthermore, observational studies are limited to identifying associations and do not offer insights into causal relationships.
In recent years, the emergence of large-scale genome-wide association studies (GWAS) has provided researchers with the opportunity to utilize genetic variations for inferring causal relationships between exposures and hypothesized outcomes. The Mendelian randomization study (MR), an innovative genetic analysis approach, has been widely applied to evaluate potential causality between exposures and clinical outcomes [14, 15]. Since genetic information is randomly inherited from parents to offspring at the time of conception, employing MR to simulate randomized clinical trials—with the most prevalent single nucleotide polymorphisms (SNPs) in genetic variations serving as instrumental variables—can effectively reduce confounding effects and thereby clarify the causality between exposure factors and outcome variables.
This study is designed to employ MR to pinpoint plasma metabolites and proteins that are causally linked to ovarian cancer. By utilizing these causally associated proteins to create a gene panel, the study aims to develop a predictive model that will enhance our understanding of the molecular mechanisms involved in the advancement of ovarian cancer and the resistance to chemotherapy. The primary objective is to identify crucial biomarkers and elucidate the fundamental processes that contribute to the onset, progression, and therapeutic resistance of ovarian cancer. The innovation and importance of this research stem from its capability to provide causally inferred biomarkers, which could greatly improve early diagnostic precision, enhance prognostic assessments, and guide personalized treatment approaches, ultimately leading to better clinical outcomes for patients with ovarian cancer.
2 Results
2.1 Confirmation of plasma metabolites associated with ovarian cancer
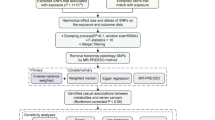
To guarantee the efficacy of instrumental variables (IVs), the chosen instrumental variables must adhere to the three fundamental assumptions of Mendelian randomization analysis. Initially, SNPs that are strongly linked to the exposure factor were selected as instrumental variables (P < 5*10–8), ensuring a lack of correlation among individual SNPs (r^2 = 0.001). Causal relationships between plasma metabolites and ovarian cancer were evaluated using MR-IVW, MR-Egger, weighted median, and weighted models. In the MR analysis concerning these plasma metabolites and ovarian cancer, a significance threshold of p < (0.05/824) was established based on the Bonferroni correction method, resulting in the identification of 9 metabolites with a p-value below this threshold. Following this, four metabolites that produced inconsistent results across the four MR models and those with an SNP count of fewer than 10 were excluded, leading to a final selection of 7 metabolites. The comprehensive analysis process and results are detailed in Supplementary material 1.
2.2 Reverse causality verification
We utilized SNPs that showed a significant association with ovarian cancer as instrumental variables to explore the possible reverse causal relationship between ovarian cancer and seven plasma metabolites. Using the IVW method for our analysis, we eliminated three plasma metabolites that displayed a reverse causal relationship with ovarian cancer (P < 0.05). Further analysis was performed on the remaining four plasma metabolites, which did not reveal any reverse causal relationship with ovarian cancer (P > 0.05). The detailed results are presented in Supplementary material 1.
2.3 Sensitivity analysis
In the course of performing sensitivity analysis, redundant SNPs were identified and excluded. The estimated outcomes of the Mendelian randomization effect are presented in Table 1, with the p-value of IVW being less than 0.05, which suggests that a significant association is still maintained.
Cochran's Q test was utilized to evaluate heterogeneity in the research findings, with no significant heterogeneity detected among the SNPs (P > 0.05). MR-Egger regression analysis was conducted to explore pleiotropy within the randomization analysis results, revealing an absence of significant pleiotropy (P > 0.05), which suggests that pleiotropy is unlikely to cause considerable bias in the findings. The MR-PRESSO test indicated no outlier SNPs and no significant horizontal pleiotropy (P > 0.05). Employing the Leave-one-out method to assess the influence of individual SNPs on the overall results demonstrated that removing any single SNP does not materially change the outcome, underscoring the robustness of the MR results.
In the context of heterogeneity, we performed additional validation through the application of the random effects model. Testing with the random effects MR model revealed that the relationship between exposure and outcome continued to be significant, with a p-value below 0.05, suggesting a causal relationship between them. The findings are presented in Table 2.
2.4 Confirmation of plasma proteins associated with ovarian cancer
To guarantee the efficacy of IVs, we meticulously followed the three fundamental assumptions of Mendelian randomization analysis. First, we identified single nucleotide polymorphisms (SNPs) that exhibit a strong association with the exposure factor as instrumental variables (P < 5*10^-8), while ensuring their independence from one another (r^2 = 0.001). Next, we utilized MR-IVW, MR-Egger, weighted median, and weighted models to thoroughly evaluate the causal links between 2,297 plasma proteins and ovarian cancer. Our Mendelian randomization analysis relating 2,297 plasma proteins to ovarian cancer pinpointed 22 plasma proteins with a significant IVW p-value (< 0.05/250). Subsequently, duplicate SNPs were detected and removed. Afterward, 14 proteins associated with fewer than 2 SNPs were excluded, resulting in a final selection of 8 proteins. The comprehensive results of the analysis are presented in Supplementary material 2.
2.5 Reverse causality verification
Utilizing SNPs that are significantly linked to ovarian cancer as instrumental variables, we carried out an analysis to investigate the possible reverse causal association between ovarian cancer and the identified 8 plasma proteins. Through the application of the IVW method for analysis, we determined that there was no reverse causal relationship with ovarian cancer (P > 0.05). We concentrated on an in-depth analysis of the 8 plasma proteins. The results of the specific analyses are presented in Supplementary material 1, while the findings from the Mendelian randomization effect are detailed in Table 3.
2.6 Sensitivity analysis
The detailed test results are presented in Table 4. In instances where heterogeneity was detected, the random effects model was employed to re-evaluate the data. Subsequent to the application of the random effects MR model, the relationship between exposure and outcome continued to be significant, with a p-value below 0.05, suggesting a causal link between the two variables.
2.7 Logistic regression and ROC curve
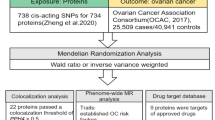
We obtained transcriptome data for ovarian cancer (GSE51373) from the GEO database, which includes 28 cases of human ovarian cancer cell lines, categorized into 12 chemoresistant and 16 chemosensitive cases. Utilizing ovarian cancer-related characteristic proteins identified from the Uniprot website based on MR analysis results, we extracted the expression matrix of pertinent feature genes from the ovarian cancer transcriptome data. Following this, we performed binary logistic regression to analyze a selection of ovarian cancer feature genes. This analysis allowed us to develop a predictive model for the disease, incorporating the expression level variations of multiple feature genes across different samples into a 0–1 predictive probability. Subsequently, we conducted multivariate disease diagnosis prediction using receiver operating characteristic (ROC) curve analysis. The AUC was determined to be 0.844 (p = 0.002; 95% CI: 0.678–1.000), demonstrating that these feature genes hold significant diagnostic and evaluative potential regarding drug resistance in ovarian cancer cells (Fig. 1). These results indicate the possibility for further investigations into the mechanisms underlying drug resistance in ovarian cancer.
2.8 Construction of PPI network and its role in ovarian cancer
To investigate the specific biological roles of the identified feature proteins in ovarian cancer, we performed a thorough analysis utilizing the STRING database, establishing a medium confidence score threshold (> 0.4), and created a network graph depicting pertinent protein interactions (Fig. 2). The analysis demonstrated that C–C motif chemokine ligand 20 (CCL20) and matrix metallopeptidase 1 (MMP1) were involved in the IL17 signaling pathway, whereas EXOSC3 was linked to the exosome-related signaling pathway. Furthermore, proteins associated with the pathways for SULT1E1, SNX7, ARL1, MAN2B2, and LYG1 may have significant roles in ovarian cancer. SULT1E1 was a critical enzyme in maintaining estrogen homeostasis, and the onset of ovarian cancer was strongly correlated with estrogen levels. SNX7 participated in the regulation of phagocytosis and the assembly of autophagosomes. ARL1 was a GTP-binding protein that contributed to a variety of essential cellular processes. MAN2B2 was part of the glycosyl hydrolase gene family and was involved in the degradation of glycoproteins within the lysosome. LYG1 belonged to the lysozyme G family and might exert anti-tumor effects by enhancing the activation, proliferation, and functionality of CD4 + T cells within the tumor microenvironment.
3 Discussion
This study utilized Mendelian randomization analysis techniques to investigate the causal links between plasma metabolites, plasma proteins, and the risk of ovarian cancer. The results indicated that four plasma metabolites (Succinylcarnitine, Triglycerides in large LDL, Triglycerides in very small VLDL, and Phospholipids in very small VLDL) and four plasma proteins (ADP-ribosylation factor-like protein 1, Lysozyme g-like protein 1, Sulfotransferase 1E1, and C–C motif chemokine ligand 20) are associated with an elevated risk of ovarian cancer. In contrast, four plasma proteins (Exosome complex component RRP40, Mannosidase alpha class 2B member 2, Sorting nexin-7, and matrix metallopeptidase 1) demonstrate a protective effect against ovarian cancer. Concurrently, we compiled relevant ovarian cancer gene datasets from the GEO database. Employing binary logistic regression, we developed a predictive model for the disease based on the plasma protein genes identified. The predictive model for ovarian cancer chemotherapy resistance, which includes eight feature genes, exhibited an AUC area of 0.844 (p = 0.002) through multivariate ROC curve analysis. The amalgamation of PPI protein network interaction diagrams and literature analysis shed light on the potential mechanisms by which these proteins may influence the occurrence and progression of ovarian cancer.
This study uncovered significant causal links between specific plasma metabolites and ovarian cancer via an extensive Mendelian randomization analysis. The metabolites identified, including Succinylcarnitine, Triglycerides in large LDL, Triglycerides in very small VLDL, and Phospholipids in very small VLDL, are all related to lipid metabolism. Recent investigations have underscored the commonality of metabolic abnormalities in tumors, where lipids are essential for forming cellular membranes, supplying energy, and producing signaling molecules for tumors [16]. Dysfunctions in lipid metabolism, which include the synthesis, degradation, and uptake of phospholipids, cholesterol, fatty acids, and triglycerides, may affect cell proliferation, invasion, metastasis, and chemotherapy resistance through the process of cancer reprogramming [17, 18]. A plethora of studies has indicated a correlation between the malignant progression of ovarian cancer and an increased lipid content along with metabolite accumulation. [19] For example, GERCEL-TAYLOR C et al. [20] noted heightened levels of HSL in the ascites of ovarian cancer patients, while Li J et al. [21] associated elevated triglycerides with a greater cancer risk among patients with high-grade serous ovarian cancer. Xu Zhang et al. conducted a meta-analysis revealing a significant positive correlation between serum triglycerides and the risk of ovarian cancer [22]. Lysophosphatidic acid, a metabolite derived from phospholipids, has demonstrated the ability to promote the growth, migration, and invasion of ovarian cancer cells [23]. Furthermore, clinical assessments of serum TG, LDL-C, and HDL-C levels in ovarian cancer patients can be instrumental in monitoring prognosis [24]. M V Iurova and collaborators also established through high-performance liquid chromatography mass spectrometry (HPLC–MS) that various lipid types (such as triglycerides) in plasma samples exhibited unique characteristics in stage I-II ovarian cancer, suggesting their potential as molecular biomarkers for early ovarian cancer prediction [25]. These findings imply that the plasma metabolites identified in this study may be intricately linked to the onset, progression, and metastasis of ovarian cancer. They have the potential to serve as essential indicators for ovarian cancer prediction, but further validation through clinical study data is warranted.
Considering that metabolites represent secondary products of proteins, this study broadened its analysis to encompass plasma proteins, uncovering significant causal relationships between particular plasma proteins and ovarian cancer. The proteins identified, specifically CCL20, SULT1E1, LYG1, ARL1, MMP1, EXOSC3, SNX7, and MAN2B2, underwent a comprehensive examination of their biological functions. It was found that these proteins predominantly regulate the IL-17 signaling pathway and pathways associated with exosomes.
The Interleukin-17 (IL-17) family, which includes IL-17 A-F, is essential for inflammatory responses and immune defense [26]. IL-17 interacts with adapter proteins Act1 and TNF receptor-related factor 6 (TRAF6) via its binding to the heterodimeric receptor complex formed by IL-17RA and IL-17RC subunits. This activation pathway subsequently triggers MAPK and NF-κB, leading to the regulation of pro-inflammatory cytokine and chemokine expression, which affects cancer progression [27]. Studies indicate a potential relationship between the IL-17 signaling pathway and chemotherapy sensitivity in patients with ovarian cancer, with downstream elements such as CCL20 and MMP1 playing a role [28]. The CCR6/CCL20 axis has been linked to several cancers, including lung cancer, colorectal cancer, ovarian cancer, and thyroid cancer [29,30,31,32]. CCR6, a G protein-coupled receptor, is crucial for cancer cell invasion and dissemination by interacting with its ligand, CCL20 [33, 34]. Cisplatin-stimulated classically activated macrophages have been shown to facilitate ovarian cancer cell migration through the CCR6/CCL20 pathway [31]. Further studies have suggested that the CCR6/CCL20 mechanism may enhance the metastasis of ovarian cancer by affecting cancer cell adhesion and the epithelial-mesenchymal transition [35]. Matrix metalloproteinases (MMPs), particularly MMP1, play a significant role in tumor development [36]. The involvement of MMP1 in the metastasis and invasion of various tumors is well established [37, 38]. It may influence tumor growth by activating the PI3/AKT signaling pathway [39]. Ovarian cancer cells have been observed to release extracellular vesicles containing MMP1 mRNA, leading to the disruption of mesothelial cells and facilitating peritoneal spread, which indicates a correlation with poor prognosis in ovarian cancer [40].
EXOSC3 (Exosome complex component RRP40), is a constituent of the RNA exosome. Research conducted by Masumi Tsuda and colleagues [41] noted a significant increase in EXOSC3 expression in the normal mucosa of colon cancer patients. This increase was linked to the activation of the ERK1/2 and JNK signaling pathways. The study proposed that the RNA stabilization facilitated by EXOSC3 may play a role in the advancement of cancer.
SULT1E1 (Sulfotransferase 1E1) is an enzyme that functions as a sulfotransferase, facilitating the sulfate conjugation of estradiol and estrone by utilizing 3'-PhosphoAdenosine-5'-PhosphoSulfate (PAPS) as the sulfonate donor. SULT1E1 plays a crucial role in maintaining estrogen homeostasis [42]. Research has shown that estrone sulfate (E1S) can convert to 17β-estradiol (E2) within tumors via the sulfatase pathway. E2 has the potential to enhance the progression of epithelial ovarian cancer. FELICITAS MUNGENAST et al. [43] proposed that SULT1E1, functioning as an estrogen-modifying enzyme within the sulfotransferase pathway, may be linked to the prognosis of ovarian cancer. This study indicated that SULT1E1 could represent a promising target for endocrine therapy in patients with ovarian cancer.
SNX7 (Sorting nexin 7) is a protein that is linked to early endosomes and multivesicular bodies. It is a member of the sorting nexin family and works alongside SNX4 to assist in autophagosome assembly and in the processing of amyloid precursor protein. This protein regulates the transport and recycling of the phospholipid scramblase ATG9A [44]. Research conducted by Jianlin Chen et al. [45] revealed elevated levels of SNX7 in liver cancer tissues, indicating a possible relationship with the immune microenvironment and its effect on tumor development and drug resistance. ARL1 (ADP-ribosylation factor-like protein 1) is a human protein that is highly homologous to aldose reductase, playing a crucial role in various essential cellular processes [46]. A study by Cheila Brito et al. [47] showed that increased expression of ARL1 led to enhanced infiltration of CD4 + T cells and neutrophils, which could potentially affect tumor progression and the immune microenvironment. MAN2B2 (Mannosidase alpha class 2B member) belongs to the glycosyl hydrolase gene family and is involved in the lysosomal degradation of glycoproteins [48]. Research by Xiaoying Fu et al. [49] found that circRNA-MAN2B2 was highly expressed in hepatocellular carcinoma (HCC) tissues, suggesting a possible link to the prognosis of liver cancer patients. LYG1 (Lysozyme g-like protein 1) is part of the lysozyme G family and may have anti-tumor effects by promoting the activation, proliferation, and function of CD4 + T cells within the tumor microenvironment [50]. Although research on LYG1 in ovarian cancer is limited, So-Yeon Lee et al. [51] proposed its potential as a reliable molecular marker for non-invasive follicular thyroid tumors.
In conclusion, the plasma metabolites and proteins that have been identified, along with the thoroughly analyzed associated signaling pathways, are expected to significantly impact the onset, development, drug resistance, and prognosis of ovarian cancer. These results have the potential to act as future biomarkers, providing a promising direction for additional experimental validation.
This research applies Mendelian randomization to independently explore the causal relationship between plasma metabolites, plasma proteins, and ovarian cancer. This methodology demonstrates superior effectiveness in managing confounding variables and reducing the impact of reverse causality compared to traditional epidemiological methods. To strengthen adherence to the three assumptions of MR, we conducted essential sensitivity analyses. Our strategy involved selecting genetic variants from GWAS data that exhibit a strong correlation with the phenotype while ensuring independence to satisfy the "association" assumption. Simultaneously, the "exclusion" assumption was upheld by omitting genetic variations associated with potential confounders. The application of various causality estimation techniques reinforced the robustness of our findings, with consistent results across different methods providing further validation. To confirm the reliability of our MR analysis results, we obtained transcriptome data for ovarian cancer cell lines from the GEO database. Utilizing logistic regression and ROC analysis, we assert that plasma proteins causally associated with ovarian cancer demonstrate significant diagnostic potential for chemotherapy resistance in ovarian cancer cell lines. This inference not only opens a new pathway for the exploration of regulatory mechanisms aimed at inhibiting the initiation, progression, and drug resistance of ovarian cancer but also presents promising advancements for early diagnosis and treatment of the condition. Furthermore, it has the capacity to improve chemotherapy sensitivity and positively influence patient outcomes.
This study presents several limitations. Firstly, the data used in this research mainly comes from European populations, which may limit the applicability of our findings to other ethnic groups. Secondly, although our analysis has created a solid theoretical framework for comprehending the onset and chemotherapy resistance associated with ovarian cancer, further experimental studies are necessary. Conducting experiments will clarify the specific roles of these proteins in the mechanisms that contribute to the development of ovarian cancer and its drug resistance. This further research is crucial to provide practical evidence for treatment strategies in clinical settings.
In conclusion, this research employed Mendelian randomization methods to investigate the causal links between plasma metabolites, plasma proteins, and ovarian cancer. The study revealed new biomarkers related to the progression of ovarian cancer, indicating that certain plasma proteins possess remarkable diagnostic potential for the chemoresistance noted in ovarian cancer cell lines. However, additional experimental validation is crucial to clarify the mechanisms that underlie these associations.
4 Methods
4.1 Data source
The data concerning the exposure factor (plasma metabolites) and the outcome (ovarian cancer) in this investigation were obtained from the IEU open GWAS database (https://gwas.mrcieu.ac.uk/). The plasma metabolite information was derived from three datasets: met-a (from So-Youn Shin et al.) [52], met-c (from Johannes Kettunen et al.) [53], and met-d (from Borges CM et al.). The ovarian cancer data were sourced from the dataset ieu-a-1235 (from Catherine M Phelan et al.) [54]. Detailed information on the total sample size for each dataset, the count of SNPs, publication years, and the respective authors is presented in Table 5. It is significant to highlight that all populations included in these datasets are of European ancestry.
The exposure factor (plasma proteins) and outcome (ovarian cancer) data for this study were obtained from the IEU open GWAS database (https://gwas.mrcieu.ac.uk/). Plasma proteins were sourced from three datasets: prot-a (from Benjamin B Sun et al.) [55], prot-b (from Lasse Folkersen et al.) [56], and prot-c (from Karsten Suhre et al.) [57]. Ovarian cancer data were extracted from the dataset: ieu-a-1234 (from Catherine M Phelan et al.) [54]. Detailed information on the total sample size of the dataset, the number of SNPs, publication years, and authors are illustrated in Table 5. The included populations are all of European ancestry.
4.2 Study design
This research utilized published GWAS data and applied bidirectional Mendelian randomization analysis to investigate the causal association between plasma metabolites, plasma proteins, and the risk of ovarian cancer.
Simultaneously, we compiled a relevant ovarian cancer gene dataset from the GEO database and employed binary logistic regression to develop a predictive model for the identified plasma protein genes. Additionally, we performed multivariate ROC curve analysis on the feature genes to evaluate their diagnostic efficacy for ovarian cancer.
The data utilized in this study were publicly accessible, with each primary study securing ethical approval and informed consent. The general design of the study is depicted in Fig. 3.
4.3 Selection of genetic instrumental variables
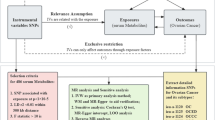
This study adhered to the three fundamental assumptions of MR [58] 1. The assumption of association: Instrumental variables (i.e., genetic variations) were strongly correlated with the exposure factor, meaning there should be a robust relationship between plasma metabolites or plasma protein SNPs and ovarian cancer; 2. The assumption of independence: No unmeasured confounding factors existed between the instrumental variables and the outcomes; 3. The assumption of exclusion: Instrumental variables could only influence the outcome through the exposure, implying no pleiotropy, such that SNPs serving as instrumental variables could only affect ovarian cancer via plasma metabolites or plasma proteins. To minimize the impact of SNP linkage disequilibrium on the analysis results, the criteria for SNP selection were set as P < 5*10^-8, linkage disequilibrium r2 < 0.001, and a genetic distance of 1000 kb. Instrumental variables strongly associated with the exposure factor and meeting these criteria were selected. For each SNP, relevant information, including major allele, allele frequency, β coefficient, p-value, and standard error, was collected, and an association analysis with the outcome was conducted.
4.4 Mendelian randomization statistical analysis
This study utilized the inverse-variance weighted method (IVW) [59], MR-Egger regression (MR-Egger) [60], and the weighted median estimator (WME) [61] to assess the causal relationships between plasma metabolites, plasma proteins, and the risk of ovarian cancer. The IVW, a conventional approach in two-sample Mendelian randomization studies, was the primary analytical method. It involved calculating the Wald ratio for each SNP (the effect size of the SNP on the outcome divided by the effect size of the SNP on the exposure). These Wald ratios were then weighted and combined to evaluate the association between the genetic prediction of exposure and the risk of the outcome. However, this method provided a consistent estimate of causal effects only if all genetic variations analyzed were valid instrumental variables. In addition to IVW, the study also used MR-Egger regression and WME as supplementary analytical methods. MR-Egger helps identify and adjust for bias due to horizontal pleiotropy, while WME can still provide relatively consistent estimated causal effects even if 50% of the instrumental variables are invalid. These supplementary methods, though less effective in detecting the true causal effect, were employed to support the findings from IVW. A significant causal estimate in IVW, along with a consistent direction in MR-Egger and WME, was deemed indicative of significance.
4.5 Reverse causality
Following the criteria for selecting instrumental variables, relevant instruments were identified from the GWAS database, with ovarian cancer serving as the exposure and plasma proteins or metabolites as the outcomes. Subsequently, a Mendelian randomization analysis was conducted to investigate reverse causality. A thorough evaluation of reverse causal effects was carried out using MR-IVW, MR-Egger, weighted median, and weighted models.
4.6 Sensitivity analysis
To strengthen the robustness of the Mendelian randomization (MR) analysis, we performed additional tests to validate the reliability of our findings. Cochran's Q test was used to evaluate the heterogeneity of the results, and it indicated no significant heterogeneity among the SNPs (P > 0.05). Additionally, MR-Egger regression analysis was applied to assess the pleiotropy of the randomization results, and the findings suggested no horizontal pleiotropy among the instrumental variables (P > 0.05). Subsequent MR-PRESSO and Leave-one-out analyses also revealed no outlier SNPs and no significant horizontal pleiotropy (P > 0.05). These results collectively support the high reliability of the MR analysis outcomes.
The MR-PRESSO method was utilized to detect outlier instrumental variables, and a re-analysis was conducted after excluding these outliers. In the sensitivity analysis, individual SNPs were removed one at a time, and the "leave-one-out" method was applied to evaluate the impact of each SNP on the results [62]. Additionally, the intercept from the MR-Egger regression was utilized to assess the horizontal pleiotropy of the SNPs. The Cochran Q test was performed to evaluate the heterogeneity of the instrumental variables, with a p-value less than 0.05 indicating the presence of heterogeneity.
4.7 Logistic regression and ROC curve
The gene names of plasma proteins associated with ovarian cancer were identified using the UniProt website (https://www.uniprot.org/), as shown in Table 3. Next, the gene expression data for ovarian cancer (GSE51373) was retrieved from the GEO database (https://www.ncbi.nlm.nih.gov/geo/), and the gene expression matrix for the relevant proteins was extracted. A disease prediction model was developed using binary logistic regression, integrating a set of ovarian cancer-related protein features into a 0–1 predictive probability. The diagnostic value of these feature genes for ovarian cancer was subsequently evaluated through multivariate ROC curve analysis.
4.8 PPI network construction and prediction of its role in ovarian cancer
The series of ovarian cancer-related proteins, after filtering, were uploaded to the STRING website (http://www.string-db.org/) [63]. Proteins that did not exhibit any interactions were excluded, and a protein–protein interaction (PPI) network was constructed using an interaction score threshold of > 0.4 for differentially expressed gene (DEG)-encoded proteins. The potential roles of these key proteins in critical regulatory signaling pathways for ovarian cancer patients were analyzed based on biological function annotations from KEGG and GO.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its Supplementary files).
Change history
03 April 2025
This article has been updated to correct the equal contribution statement.
Abbreviations
- GWAS:
-
Genome-wide association studies
- MR:
-
Mendelian randomization
- SNPs:
-
Single nucleotide polymorphisms
- IVs:
-
Instrumental variables
- CCL20:
-
C–C motif chemokine ligand 20
- MMP1:
-
Matrix metallopeptidase 1
- EXOSC3:
-
Exosome complex component RRP40
- SULT1E1:
-
Sulfotransferase 1E1
- SNX7:
-
Sorting nexin-7
- ARL1:
-
ADP-ribosylation factor-like protein 1
- LYG1:
-
Lysozyme g-like protein 1
- MAN2B2:
-
Mannosidase alpha class 2B member 2
- ROC:
-
Receiver operating characteristic
- IL-17:
-
Interleukin-17
- TRAF6:
-
TNF receptor-related factor 6
- PAPS:
-
3'-PhosphoAdenosine-5'-PhosphoSulfate
- E1S:
-
Estrone sulfate
- E2:
-
17β-Estradiol
- HCC:
-
Hepatocellular carcinoma
References
Lheureux S, et al. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–53.
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Torre LA, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–96.
Matulonis UA, et al. Ovarian cancer. Nat Rev Dis Prim. 2016;2:16061.
Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Menon U, Griffin M, Gentry-Maharaj A. Ovarian cancer screening–current status, future directions. Gynecol Oncol. 2014;132(2):490–5.
Bast RC Jr, et al. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68(5):1331–7.
Moss EL, Hollingworth J, Reynolds TM. The role of CA125 in clinical practice. J Clin Pathol. 2005;58(3):308–12.
Moore RG, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108(2):402–8.
Trabert B, et al. Ovarian cancer risk in relation to blood cholesterol and triglycerides. Cancer Epidemiol Biomarkers Prev. 2021;30(11):2044–51.
Zhu Y, et al. Carnitine palmitoyltransferase 1A promotes mitochondrial fission by enhancing MFF succinylation in ovarian cancer. Commun Biol. 2023;6(1):618.
Yagi T, et al. Relative ratios enhance the diagnostic power of phospholipids in distinguishing benign and cancerous ovarian masses. Cancers. 2019. https://doi.org/10.3390/cancers12010072.
Shu NH. Characteristics and clinical significance of the time domain and frequency domain parameters of heart sounds in patients with hypertension. Zhonghua Yi Xue Za Zhi. 1988;68(4):201–4.
Frayling TM, Stoneman CE. Mendelian randomisation in type 2 diabetes and coronary artery disease. Curr Opin Genet Dev. 2018;50:111–20.
Lawlor DA, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Zhao G, Cardenas H, Matei D. Ovarian cancer-why lipids matter. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11121870.
Zhang C, Quinones A, Le A. Metabolic reservoir cycles in cancer. Semin Cancer Biol. 2022;86(Pt 3):180–8.
Shan L, et al. Measurement of phospholipids may improve diagnostic accuracy in ovarian cancer. PLoS ONE. 2012;7(10): e46846.
Lin Q, et al. Associations of preoperative serum high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels with the prognosis of ovarian cancer. Arch Gynecol Obstet. 2022;305(3):683–91.
Gercel-Taylor C, et al. Aberrations in normal systemic lipid metabolism in ovarian cancer patients. Gynecol Oncol. 1996;60(1):35–41.
Li J, et al. Distinct plasma lipids profiles of recurrent ovarian cancer by liquid chromatography-mass spectrometry. Oncotarget. 2017;8(29):46834–45.
Zhang X, et al. Dietary fats and serum lipids in relation to the risk of ovarian cancer: a meta-analysis of observational studies. Front Nutr. 2023;10:1153986.
Pustilnik TB, et al. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999;5(11):3704–10.
Zhang Y, et al. High resolution mass spectrometry coupled with multivariate data analysis revealing plasma lipidomic alteration in ovarian cancer in Asian women. Talanta. 2016;150:88–96.
Iurova MV, et al. Lipid alterations in early-stage high-grade serous ovarian cancer. Front Mol Biosci. 2022;9: 770983.
Qian Y, et al. IL-17 signaling in host defense and inflammatory diseases. Cell Mol Immunol. 2010;7(5):328–33.
Ma X, et al. Hippo kinase NDR2 inhibits IL-17 signaling by promoting Smurf1-mediated MEKK2 ubiquitination and degradation. Mol Immunol. 2019;105:131–6.
Zheng H, et al. Identification of the key genes associated with chemotherapy sensitivity in ovarian cancer patients. Cancer Med. 2020;9(14):5200–9.
Kirshberg S, et al. Involvement of CCR6/CCL20/IL-17 axis in NSCLC disease progression. PLoS ONE. 2011;6(9): e24856.
Frick VO, et al. Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal malignancy: an overview. World J Gastroenterol. 2016;22(2):833–41.
Liu W, et al. Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett. 2020;472:59–69.
Zeng W, et al. CCL20/CCR6 promotes the invasion and migration of thyroid cancer cells via NF-kappa B signaling-induced MMP-3 production. Exp Mol Pathol. 2014;97(1):184–90.
Furue K, et al. The CCL20 and CCR6 axis in psoriasis. Scand J Immunol. 2020;91(3): e12846.
Gómez-Melero S, Caballero-Villarraso J. CCR6 as a potential target for therapeutic antibodies for the treatment of inflammatory diseases. Antibodies (Basel). 2023. https://doi.org/10.3390/antib12020030.
Liu W, et al. The role of CCL20-CCR6 axis in ovarian cancer metastasis. Onco Targets Ther. 2020;13:12739–50.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67.
Bao W, et al. HER2-mediated upregulation of MMP-1 is involved in gastric cancer cell invasion. Arch Biochem Biophys. 2010;499(1–2):49–55.
Park YH, et al. Ets-1 upregulates HER2-induced MMP-1 expression in breast cancer cells. Biochem Biophys Res Commun. 2008;377(2):389–94.
Liu M, et al. MMP1 promotes tumor growth and metastasis in esophageal squamous cell carcinoma. Cancer Lett. 2016;377(1):97–104.
Yokoi A, et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470.
Tsuda M, et al. Aberrant expression of MYD88 via RNA-controlling CNOT4 and EXOSC3 in colonic mucosa impacts generation of colonic cancer. Cancer Sci. 2021;112(12):5100–13.
Hempel N, et al. Site-directed mutagenesis of the substrate-binding cleft of human estrogen sulfotransferase. Biochem Biophys Res Commun. 2000;276(1):224–30.
Mungenast F, et al. Clinical significance of the estrogen-modifying enzymes steroid sulfatase and estrogen sulfotransferase in epithelial ovarian cancer. Oncol Lett. 2017;13(6):4047–54.
Antón Z, et al. A heterodimeric SNX4–SNX7 SNX-BAR autophagy complex coordinates ATG9A trafficking for efficient autophagosome assembly. J Cell Sci. 2020. https://doi.org/10.1242/jcs.246306.
Chen J, et al. Comprehensive analysis and validation of SNX7 as a novel biomarker for the diagnosis, prognosis, and prediction of chemotherapy and immunotherapy response in hepatocellular carcinoma. BMC Cancer. 2023;23(1):899.
Cao D, Fan ST, Chung SS. Identification and characterization of a novel human aldose reductase-like gene. J Biol Chem. 1998;273(19):11429–35.
Brito C, et al. Unraveling the relevance of ARL GTPases in cutaneous melanoma prognosis through integrated bioinformatics analysis. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22179260.
Verheijen J, et al. Defining a new immune deficiency syndrome: MAN2B2-CDG. J Allergy Clin Immunol. 2020;145(3):1008–11.
Fu X, et al. Circular RNA MAN2B2 promotes cell proliferation of hepatocellular carcinoma cells via the miRNA-217/MAPK1 axis. J Cancer. 2020;11(11):3318–26.
Liu H, et al. LYG1 exerts antitumor function through promoting the activation, proliferation, and function of CD4(+) T cells. Oncoimmunology. 2017;6(4): e1292195.
Lee SY, et al. Identification of NIFTP-specific mRNA markers for reliable molecular diagnosis of thyroid tumors. Endocr Pathol. 2023;34(3):311–22.
Shin SY, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46(6):543–50.
Kettunen J, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016;7:11122.
Phelan CM, et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet. 2017;49(5):680–91.
Sun BB, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73–9.
Folkersen L, et al. Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLoS Genet. 2017;13(4): e1006706.
Suhre K, et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun. 2017;8:14357.
Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj. 2018. https://doi.org/10.1136/bmj.k601.
Burgess S, et al. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Bowden J, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted Median estimator. Genet Epidemiol. 2016;40(4):304–14.
Bo L, Wang L, Jiao L. Feature scaling for kernel fisher discriminant analysis using leave-one-out cross validation. Neural Comput. 2006;18(4):961–78.
Szklarczyk D, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362-d368.
Acknowledgements
Not applicable.
Funding
There was no project specific funding for this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Writing—original draft preparation: [Manyin Zhao, Lin Qi, Jingjing Ren]; Writing—review and editing: [Lin Qi, Cheng Zhang, Yinuo Liu]; Conceptualization: [Lin Qi, Manyin Zhao, Yinuo Liu]; Methodology: [Cheng Zhang, Jingjing Ren, Yinuo Liu]; Formal analysis and investigation: [Lin Qi, Cheng Zhang, Wenshu Li]; Resources: [Manyin Zhao, Jingjing Ren]; Supervision: [Manyin Zhao, Jingjing Ren, Cheng Zhang],and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All analyses were based on previously published studies, and therefore, no ethical approval or patient consent was required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qi, L., Zhang, C., Liu, Y. et al. Plasma proteomes and metabolism with genome-wide association data for causal effect identification in ovarian cancer. Discov Onc 16, 388 (2025). https://doi.org/10.1007/s12672-025-02087-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-025-02087-0