Abstract
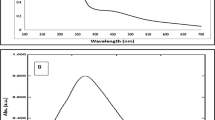
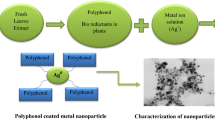
Areca catechu seed, often known as areca nut, is a source of numerous phytochemicals and is one of the main ingredients in the preparation of betel. In this study, silver nanoparticles (AgNPs) have been fabricated by a microwave irradiation system using A. catechu seed extract as an effective bioreductant. The antioxidant efficacy of A. catechu seed extract was demonstrated using DPPH radical scavenging, ferric ion, hydrogen peroxide radical, and nitric oxide radical scavenging assay. The AgNPs were found to be spherical in shape, with an average particle size of 17.5 ± 0.5 nm, as evident by HRTEM analysis. AgNPs were tested for antibacterial effectiveness against multidrug-resistant Escherichia coli (MLD 2), Klebsiella oxytoca (MLD 41), and Staphylococcus aureus (MLD 4) bacterial strains using standard antimicrobial assays including MIC, MBC, agar diffusion, and biofilm reduction assay. It was observed that MLD 41 (MIC value 12.5 μg/ml) appeared as the most sensitive against AgNPs, followed by MLD 2 (MIC value 50 μg/ml) and MLD 4 (MIC value 50 μg/ml). AgNPs were also found to be biocompatible (up to 50 μg/ml of AgNPs), as evident by the hemolysis assay. This study shows a simple, eco-friendly technique for fabricating AgNPs from A. catechu seed extract, as well as its antibacterial capabilities.












Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- XRD:
-
X-ray powder diffraction
- UV–vis:
-
Ultraviolet–visible spectroscopy
- SAED:
-
Selected area (electron) diffraction
- PBS:
-
Phosphate buffer saline
- NB:
-
Nutrient broth
- NA:
-
Nutrient agar
- MLD 41:
-
Klebsiella oxytoca
- MLD 4:
-
Staphylococcus aureus
- MLD 2:
-
Escherichia coli
- MIC:
-
Minimum inhibitory concentration
- MBC:
-
Minimum bactericidal concentration
- HRTEM:
-
High-resolution transmission electron microscopy
- H2O2 :
-
Hydrogen peroxide
- FTIR:
-
Fourier-transform infrared spectroscopy
- EDX:
-
Energy-dispersive X-ray analysis
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- DAD:
-
Disc agar diffusion
- AR:
-
Analytical reagent
- AgNPs:
-
Silver nanoparticles
- AgNO3 :
-
Silver nitrate
References
C Wang X Gao Z Chen Y Chen H Chen 2017 Preparation, characterization and application of polysaccharide-based metallic nanoparticles: A review Polymers 9 689
S Fazal A Jayasree S Sasidharan M Koyakutty SV Nair D Menon 2014 Green synthesis of anisotropic gold nanoparticles for photothermal therapy of cancer ACS Applied Materials & Interfaces 6 11 8080 8089
A Genevieve M Kahrilas Laura SJ Wally H Fredrick Michael L Amy JE Prieto Owens. 2014 Microwave-assisted green synthesis of silver nanoparticles using orange peel extract ACS Sustainable Chemistry & Engineering 2 3 367 376
ST Fardood A Ramazani 2016 Green synthesis and characterization of copper oxide nanoparticles using coffee powder extract Journal Nanostructures 6 2 167 171
CH Ramamurthy KS Sampath P Arunkumar Suresh kumar, M., Sujatha, V., Premkumar, K., & Thirunavukkarasu, C. 2013 Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells Bioprocess and Biosystems Engineering 36 8 1131 1139
D Suresh PC Nethravathi H Udayabhanu H Rajanaika H Nagabhushana SC Sharma 2015 Green synthesis of multifunctional zinc oxide nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities Materials Science in Semiconductor Processing 31 446 454
H Sugimoto M Fujii K Imakita 2016 Silicon nanocrystal-noble metal hybrid nanoparticles Nanoscale 8 10956 10962
SK Kulkarni 2007 Nanotechnology: Principles and practices Capital Publishing Company
L Rodrı L Rodríguez-Sánchez MC Blanco LC Ferracin 2000 Electrochemical synthesis of silver nanoparticles The Journal of Physical Chemistry B 104 9683 9688
R Arif R Uddin 2021 A review on recent developments in the biosynthesis of silver nanoparticles and its biomedical applications Med Devices Sens. 4 e10158
S Ghotekar H Dabhane S Pansambal R Oza P Tambade V Medhane 2020 A review on biomimetic synthesis of Ag2O nanoparticles using plant extract, characterization and its recent applications Advanced Journal of Chemistry-Section B. 2 3 102 111
J Davies D Davies 2010 Origins and evolution of antibiotic resistance Microbiology and molecular biology reviews 74 3 417 433
Yousefi, M., Dadashpour, M., Hejazi, M., Hasanzadeh, M., Behnam, B., de la Guardia, M., ... & Mokhtarzadeh, A. (2017). Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Materials Science and Engineering: C, 74, 568-581.
B Ramalingam T Parandhaman SK Das 2016 Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa ACS applied materials & interfaces 8 7 4963 4976
P Thuesombat S Hannongbua S Akasit S Chadchawan 2014 Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth Ecotoxicology and environmental safety 104 302 309
S Bangale S Ghotekar 2019 Bio-fabrication of Silver nanoparticles using Rosa Chinensis L. extract for antibacterial activities International Journal of Nano Dimension. 10 2 217 24
S Ghotekar A Savale 2018 Pansambal S. Phytofabrication of fluorescent silver nanoparticles from Leucaena leucocephala L. leaves and their biological activities Journal of Water and Environmental Nanotechnology 3 2 95 105
S Ghotekar S Pansambal SP Pawar T Pagar R Oza S Bangale 2019 Biological activities of biogenically synthesized fluorescent silver nanoparticles using Acanthospermum hispidum leaves extract SN Applied Sciences 1 11 1 2
Ghotekar, S., Pagar, K., Pansambal, S., Murthy, H. A., Oza, R. (2021) Biosynthesis of silver sulfide nanoparticle and its applications. In Handbook of greener synthesis of nanomaterials and compounds. 191–200).
Das, B., Dash, S. K., Mandal, D., Ghosh, T., Chattopadhyay, S., Tripathy, S., ... & Roy, S. (2017). Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arabian Journal of Chemistry, 10(6), 862-876.
S Agnihotri S Mukherji S Mukherji 2014 Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy Rsc Advances 4 8 3974 3983
AA Baby K Regi Raphael 2014 Potential antimicrobial, anthelmintic and antioxidant properties of Areca nut L. root Int. J. Pharm. Pharmaceut. Sci. 6 6 486 489
Samanta S, Banerjee J, Ali KM, Giri B, Dash SK. (2020). Biochemical characterization and antibiotic sensitivity profiling of uropathogenic bacterial strains isolated from urine sample of UTI patients. Journal of Advance Scientific Research.; 11 (1) suppl 1: 280–295.
RP Matamane MK Pillai S Magama 2019 DPPH radical scavenging activity of extracts from Buddleja salviifolia Pharmacology OnLine 2 233 240
N Loganayaki P Siddhuraju S Manian 2013 Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L Journal of food science and technology 50 4 687 695
Dash, S. S., Samanta, S., Dey, S., Giri, B., & Dash, S. K. (2020). Rapid green synthesis of biogenic silver nanoparticles using Cinnamomum tamala leaf extract and its potential antimicrobial application against clinically isolated multidrug-resistant bacterial strains. Biological trace element research, 198(2).
K Pavithra S Vadivukkarasi 2015 Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn Food Science and Human Wellness 4 1 42 46
CLSI (2019) In: Performance standards for antimicrobial susceptibility testing. 29th ed. Wayne, PA: Clinical and Laboratory Standards Institute; CLSI supplement M100.
Das, B., Mandal, D., Dash, S. K., Chattopadhyay, S., Tripathy, S., Dolai, D. P., ... & Roy, S. (2016). Eugenol provokes ROS-mediated membrane damage-associated antibacterial activity against clinically isolated multidrug-resistant Staphylococcus aureus strains. Infectious Diseases: Research and Treatment, 9, IDRT-S31741.
D Mandal SK Dash B Das S Chattopadhyay T Ghosh D Das S Roy 2016 Bio-fabricated silver nanoparticles preferentially targets Gram positive depending on cell surface charge Biomedicine & Pharmacotherapy 83 548 558
FA Qais I Ahmad M Altaf SH Alotaibi 2021 Biofabrication of gold nanoparticles using Capsicum annuum extract and its antiquorum sensing and antibiofilm activity against bacterial pathogens ACS Omega 6 16670 16682
Dash, S. K., Dash, S. S., Chattopadhyay, S., Ghosh, T., Tripathy, S., Mahapatra, S. K., ... & Roy, S. (2015). Folate decorated delivery of self assembled betulinic acid nano fibers: a biocompatible anti-leukemic therapy. RSC Advances, 5(31), 24144-24157.
MS Blois 1958 Antioxidant determinations by the use of a stable free radical Nature 181 4617 1199 1200
AA Dehpour MA Ebrahimzadeh NS Fazel NS Mohammad 2009 Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition Grasasy aceites 60 4 405 412
LM Sari GP Subita EI Auerkari 2017 Potential antioxidant and cytotoxic activities of areca nut (Areca catechu Linn.) extract in human oral squamous cell carcinoma and keratinocyte cells Asian J Pharm Clin Res 10 10 286 291
S Meir J Kanner B Akiri S Philosoph-Hadas 1995 Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves Journal of agricultural and food chemistry 43 7 1813 1819
SM Nabavi MA Ebrahimzadeh SF Nabavi M Fazelian B Eslami 2009 In vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrus boissieriana growing in Iran Pharmacognosy magazine 5 18 122
S Kumar A Yadav M Yadav JP Yadav 2017 Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm. f BMC research notes 10 1 1 12
A Benslama A Harrar 2016 Free radicals scavenging activity and reducing power of two Algerian Sahara medicinal plants extracts International Journal of Herbal Medicine 4 6 158 161
Parejo, I., Viladomat, F., Bastida, J., Rosas-Romero, A., Saavedra, G., Murcia, M. A., ... & Codina, C. (2003). Investigation of Bolivian plant extracts for their radical scavenging activity and antioxidant activity. Life Sciences, 73(13), 1667-1681.
MA Ebrahimzadeh SF Nabavi SM Nabavi 2009 Antioxidant activities of methanol extract of Sambucus ebulus L. flower Pakistan journal of biological sciences: PJBS 12 5 447 450
K AsokKumar M UmaMaheswari AT Sivashanmugam V SubhadraDevi N Subhashini TK Ravi 2009 Free radical scavenging and antioxidant activities of Glinus oppositifolius (carpet weed) using different in vitro assay systems Pharmaceutical Biology 47 6 474 482
F Parrino S Livraghi E Giamello R Ceccato L Palmisano 2020 Role of hydroxyl, superoxide, and nitrate radicals on the fate of bromide ions in photocatalytic TiO2 suspensions ACS Catalysis 10 14 7922 7931
Boora, F., Chirisa, E., & Mukanganyama, S. (2014). Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. Journal of Food Processing, 2014.
JB Habu BO Ibeh 2015 In vitro antioxidant capacity and free radical scavenging evaluation of active metabolite constituents of Newbouldia laevis ethanolic leaf extract Biological Research 48 1 1 10
SA Adebayo M Ondua LJ Shai SL Lebelo 2019 Inhibition of nitric oxide production and free radical scavenging activities of four South African medicinal plants Journal of inflammation research 12 195
M Bhagat R Anand R Datt V Gupta S Arya 2019 Green synthesis of silver nanoparticles using aqueous extract of Rosa brunonii Lindl and their morphological, biological and photocatalytic characterizations Journal of Inorganic and Organometallic Polymers and Materials 29 3 1039 1047
A Rangayasami K Kannan S Joshi M Subban 2020 Bioengineered silver nanoparticles using Elytraria acaulis (L.f.) Lindau leaf extract and its biological applications Biocatalysis and agricultural biotechnology 27 101690 https://doi.org/10.1016/j.bcab.2020.101690
Palani, G., Kannan, K., Radhika, D., Vijayakumar, P., & Pakiyaraj, K. (2020). Bioengineered metal and metal oxide nanoparticles for photocatalytic and biological applications: a review. Physics and Chemistry of Solid State, 21(4), 571-583. https://doi.org/10.15330/pcss.21.4.571-583.
P Bindu S Thomas 2014 Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis J Theor Appl Phys 8 123 134
RR Vanimakhal BS Ezhilarasi 2016 Phytochemical qualitative analysis and total tannin content in the aqueous extract of Areca catechu nut Asian J Biomed Pharmaceutic Sci 6 54 7 9
Yun’an Qing, L. C., Li, R., Liu, G., Zhang, Y., Tang, X., Wang, J., … & Qin, Y. 2018 Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies International journal of nanomedicine 13 3311
YY Loo Y Rukayadi MAR Nor-Khaizura CH Kuan BW Chieng M Nishibuchi S Radu 2018 In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens Frontiers in microbiology 9 1555
A Murei K Pillay P Govender N Thovhogi WM Gitari A Samie 2021 Synthesis, characterization and in vitro antibacterial evaluation of Pyrenacantha grandiflora conjugated silver nanoparticles Nanomaterials 11 6 1568
A Sharma VK Gupta R Pathania 2019 Efflux pump inhibitors for bacterial pathogens: From bench to bedside The Indian journal of medical research 149 2 129
MA Raza Z Kanwal A Rauf AN Sabri S Riaz S Naseem 2016 Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes Nanomaterials 6 4 74
L Wang C Hu L Shao 2017 The antimicrobial activity of nanoparticles: Present situation and prospects for the future International journal of nanomedicine 12 1227
J Talapko T Matijević M Juzbašić A Antolović-Požgain I Škrlec 2020 Antibacterial activity of silver and its application in dentistry, cardiology and dermatology Microorganisms 8 9 1400
S Makkar A Aggarwal S Pasricha I Kapur 2014 To evaluate the antibacterial properties of silver nano particle based irrigant as endodontic root canal irrigant Int J Dent Health Sci 1 4 485 492
Garibo, D., Borbón-Nuñez, H. A., de León, J. N. D., Mendoza, E. G., Estrada, I., Toledano-Magaña, Y., ... & Susarrey-Arce, A. (2020). Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Scientific reports, 10(1), 1-11.
Trastoy, R., Manso, T., Fernández-García, L., Blasco, L., Ambroa, A., Perez Del Molino, M. L., ... & Tomás, M. (2018). Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clinical microbiology reviews, 31(4), e00023-18.
M Ijaz M Zafar T Iqbal 2020 Green synthesis of silver nanoparticles by using various extracts: A review Inorganic and Nano-Metal Chemistry 51 5 744 755
Buszewski, B., Railean-Plugaru, V., Pomastowski, P., Rafińska, K., Szultka-Mlynska, M., Golinska, P., ... & Dahm, H. (2018). Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. Journal of microbiology, immunology and infection, 51(1), 45-54.
YN Slavin J Asnis UO Häfeli H Bach 2017 Metal nanoparticles: Understanding the mechanisms behind antibacterial activity Journal of nanobiotechnology 15 1 1 20
McNeilly, O., Mann, R., Hamidian, M., & Gunawan, C. (2021). Emerging concern for silver nanoparticle resistance in Acinetobacter baumannii and other bacteria. Frontiers in Microbiology, 12.
DH Limoli CJ Jones DJ Wozniak 2015 Bacterial extracellular polysaccharides in biofilm formation and function Microbiology spectrum 3 3 3 3
R Roy M Tiwari G Donelli V Tiwari 2018 Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action Virulence 9 1 522 554
K Kalishwaralal S BarathManiKanth SRK Pandian V Deepak S Gurunathan 2010 Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis Colloids and Surfaces B: Biointerfaces 79 2 340 344
S Gurunathan JW Han DN Kwon JH Kim 2014 Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria Nanoscale research letters 9 1 1 17
E Park J Yi Y Kim K Choi K Park 2010 Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism Toxicol Vitr. 24 872 878
SR Goswami T Sahareen M Singh S Kumar 2015 Role of biogenic silver nanoparticles in disruption of cell–cell adhesion in Staphylococcus aureus and Escherichia coli biofilm Journal of Industrial and Engineering Chemistry 26 73 80
K Kannan D Radhika D Gnanasangeetha SK Lakkaboyana KK Sadasivuni K Gurushankar MM Hanafiah 2021 Photocatalytic and antimicrobial properties of microwave synthesized mixed metal oxide nanocomposite Inorganic Chemistry Communications 125 108429
K Kannan D Radhika AS Nesaraj KK Sadasivuni LS Krishna 2020 Facile synthesis of NiO-CYSO nanocomposite for photocatalytic and antibacterial applications Inorganic Chemistry Communications 122 108307
K Kannan D Radhika MP Nikolova V Andal KK Sadasivuni LS Krishna 2020 Facile microwave-assisted synthesis of metal oxide CdO-CuO nanocomposite: photocatalytic and antimicrobial enhancing properties Optik 218 165112
K Kannan D Radhika KR Reddy AV Raghu KK Sadasivuni G Palani K Gurushankar 2021 Gd3+ and Y3+ co-doped mixed metal oxide nanohybrids for photocatalytic and antibacterial applications Nano Express 2 1 010014
TC Dakal A Kumar RS Majumdar V Yadav 2016 Mechanistic basis of antimicrobial actions of silver nanoparticles Frontiers in microbiology 7 1831
T Ishida 2018 Antibacterial mechanism of Ag+ ions for bacteriolyses of bacterial cell walls via peptidoglycan autolysins, and DNA damages MOJ Toxicol 4 5 345 350
YH Hsueh KS Lin WJ Ke CT Hsieh CL Chiang DY Tzou ST Liu 2015 The antimicrobial properties of silver nanoparticles in Bacillus subtilis are mediated by released Ag+ ions PloS one 10 12 e0144306
K Karthik S Dhanuskodi C Gobinath S Prabukumar S Sivaramakrishnan 2019 Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance Journal of Photochemistry and Photobiology B: Biology 190 8 20
K Karthik S Dhanuskodi C Gobinath S Prabukumar S Sivaramakrishnan 2018 Nanostructured CdO-NiO composite for multifunctional applications Journal of Physics and Chemistry of Solids 112 106 118
O Długosz K Szostak A Staroń J Pulit-Prociak M Banach 2020 Methods for reducing the toxicity of metal and metal oxide NPs as biomedicine Materials 13 2 279
Wang, J., Li, J., Guo, G., Wang, Q., Tang, J., Zhao, Y., ... & Zhang, X. (2016). Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: new insight into the antimicrobial action of silver. Scientific reports, 6(1), 1-16.
A Karuppaiah K Siram D Selvaraj M Ramasamy D Babu V Sankar 2020 Synergistic and enhanced anticancer effect of a facile surface modified non-cytotoxic silver nanoparticle conjugated with gemcitabine in metastatic breast cancer cells Materials Today Communications 23 100884
B Reidy A Haase A Luch KA Dawson I Lynch 2013 Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications Materials 6 6 2295 2350
Acknowledgements
The University of Gour Banga is greatly acknowledged for providing me with the opportunity to work in the Department of Physiology and conduct biological research.
Author information
Authors and Affiliations
Contributions
Shib Shankar Dash, Sandeep Kumar Dash and Biplab Giri contributed to the study conception and design. Material preparation, data collection and analysis were performed by Shib Shankar Dash, Jhimli Banerjee, Sovan Samanta and Sandeep Kumar Dash. The first draft of the manuscript was written by Jhimli Banerjee, Sovan Samanta and Shib Shankar Dash. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Research Involving Humans and Animals Statement
The study protocol was approved by Institutional Ethics committee (Human) of the University of Gour Banga, Malda, with approval no: UGB/IEC (Human)/004–19.
Informed Consent
EDTA-stabilized human blood samples were collected from six healthy participants after getting their verbal and written consent for this study.
Consent for Publication
Manuscript is approved by all authors for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dash, S.S., Banerjee, J., Samanta, S. et al. Microwave-Assisted Fabrication of Silver Nanoparticles Utilizing Seed Extract of Areca catechu with Antioxidant Potency and Evaluation of Antibacterial Efficacy Against Multidrug Resistant Pathogenic Bacterial Strains. BioNanoSci. 12, 210–227 (2022). https://doi.org/10.1007/s12668-021-00927-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-021-00927-1