Abstract
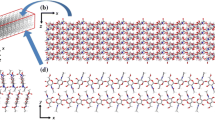
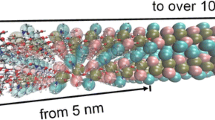
The mechanical behaviors of chitin, proteins, and their interfaces are important factors in defining the overall mechanical properties of “chitin-based” biological materials, including lobster shells, squid beaks, and spider’s fangs. Additional effects arise from their solvent environments such as water and inorganic ions. In this paper, we explored the molecular-level mechanics of the chitin–protein interface by performing molecular dynamics simulations. Model proteins including α-helices and β-sheets were investigated, showing secondary structure-dependent chitin-binding behaviors through hydrogen bonds (H-bonds). The results indicate that the terminals of proteins anchor them on the chitin substrate through H-bonds and contribute to the interfacial strength. Furthermore, it is shown that the presence of water at the interface reduces its strength by weakening the H-bonds network (by approximately two thirds for the α-helix in our model). The results and conclusion from this simple model for the chitin–protein interface are expected to shed some light on the complete exploration of multiscale mechanics in biological materials with such type of interfaces.







Similar content being viewed by others
References
Sachs, C., Fabritius, H., & Raabe, D. (2008). Influence of microstructure on deformation anisotropy of mineralized cuticle from the lobster Homarus americanus. Journal of Structural Biology, 161(2), 120–132.
Fabritius, H.-O., Sachs, C., Triguero, P. R., & Roobe, D. (2009). Influence of structural principles on the mechanics of a biological fiber-based composite material with hierarchical organization: The exoskeleton of the lobster Homarus americanus. Advanced Materials, 21(4), 391–400.
Nikolov, S., Petrov, M., Lymperakis, L., Friak, M., Sachs, C., Fabritius, H.-O., et al. (2010). Revealing the design principles of high-performance biological composites using ab initio and multiscale simulations: The example of lobster cuticle. Advanced Materials, 22(4), 519–526.
Politi, Y., Priewasser, M., Pippel, E., Zaslansky, P., Hartmann, J., Siegel, S., et al. (2012). A spider’s fang: How to design an injection needle using chitin-based composite material. Advanced Functional Materials, 22(12), 2519–2528.
Miserez, A., Schneberk, T., Sun, C., Zok, F. W., & Waite, J. H. (2008). The transition from stiff to compliant materials in squid beaks. Science, 319(5871), 1816–1819.
Gordon, L. M., & Joester, D. (2011). Nanoscale chemical tomography of buried organic–inorganic interfaces in the chiton tooth. Nature, 469(7329), 194–197.
Miserez, A., Rubin, D., & Waite, J. H. (2010). Cross-linking chemistry of squid beak. Journal of Biological Chemistry, 285(49), 38115–38124.
Atkins, E. (1985). Conformations in polysaccharides and complex carbohydrates. Journal of Biosciences, 8(1–2), 375–387.
Vincent, J. F. V., & Wegst, U. G. K. (2004). Design and mechanical properties of insect cuticle. Arthropod Structure & Development, 33(3), 187–199.
Qin, Z., Gautieri, A., Nair, A. K., Inbar, H., & Buehler, M. J. (2012). Thickness of hydroxyapatite nanocrystal controls mechanical properties of the collagen–hydroxyapatite interface. Langmuir, 28(4), 1982–1992.
Qin, Z., & Buehler, M. J. (2012). Molecular mechanics of dihydroxyphenylalanine at a silica interface. Applied Physics Letters, 101(8), 083702.
Liu, Y., Xie, B., Zhang, Z., Zheng, Q., & Xu, Z. (2012). Mechanical properties of graphene papers. Journal of the Mechanics and Physics of Solids, 60(4), 591–605.
Zeng, J.-B., He, Y.-S., Li, S.-L., & Wang, Y.-Z. (2012). Chitin whiskers: An overview. Biomacromolecules, 13(1), 1–11.
Jayakumar, R., Chennazhi, K. P., Srinivasan, S., Nair, S. V., Furuike, T., & Tamura, H. (2011). Chitin scaffolds in tissue engineering. International Journal of Molecular Sciences, 12(3), 1876–1887.
Nova, A., Keten, S., Pugno, N. M., Redaelli, A., & Buehler, M. J. (2010). Molecular and nanostructural mechanisms of deformation, strength and toughness of spider silk fibrils. Nano Letters, 10(7), 2626–2634.
Keten, S., Xu, Z., Ihle, B., & Buehler, M. J. (2010). Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk. Nature Materials, 9(4), 359–367.
Guvench, O., Greene, S. N., Kamath, G., Brady, J. W., Venable, R. M., Pastor, R. W., et al. (2008). Additive empirical force field for hexopyranose monosaccharides. Journal of Computational Chemistry, 29(15), 2543–2564.
Guvench, O., Hatcher, E., Venable, R. M., Pastor, R. W., & MacKerell, A. D., Jr. (2009). CHARMM: Additive all-atom force field for glycosidic linkages between hexopyranoses. Journal of Chemical Theory and Computation, 5(9), 2353–2370.
Guvench, O., Mallajosyula, S. S., Raman, E. P., Hatcher, E., Vanommeslaeghe, K., Foster, T. J., et al. (2011). CHARMM: Additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate–protein modeling. Journal of Chemical Theory and Computation, 7(10), 3162–3180.
MacKerell, A. D., Jr., Bashford, D., Bellott, M., Dunbrack, R. L., Jr., Evanseck, J., Field, M., et al. (1998). All-atom empirical potential for molecular modeling and dynamics studies of proteins. Journal of Physical Chemistry B, 102(18), 3586–3616.
Sikorski, P., Hori, R., & Wada, M. (2009). Revisit of alpha-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules, 10(5), 1100–1105.
Park, S., Khalili-Araghi, F., Tajkhorshid, E., & Schulten, K. (2003). Free energy calculation from steered molecular dynamics simulations using Jarzynski’s equality. Journal of Chemical Physics, 119(6), 3559–3566.
Nishiyama, Y., Noishiki, Y., & Wada, M. (2011). X-ray structure of anhydrous beta-chitin at 1 angstrom resolution. Macromolecules, 44(4), 950–957.
Beckham, G. T., & Crowley, M. F. (2011). Examination of the alpha-chitin structure and decrystallization thermodynamics at the nanoscale. Journal of Physical Chemistry B, 115(15), 4516–4522.
Plimpton, S. (1995). Fast parallel algorithms for short-range molecular-dynamics. Journal of Computational Physics, 117(1), 1–19.
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W., & Klein, M. L. (1983). Comparison of simple potential functions for simulating liquid water. Journal of Chemical Physics, 79(2), 926–935.
Ruiz, L., & Keten, S. (2011). Atomistic modeling and mechanics of self-assembled organic nanotubes. International Journal of Applied Mechanics, 3(4), 667–684.
Walton, E. B., Lee, S., & Van Vliet, K. J. (2008). Extending Bell’s model: How force transducer stiffness alters measured unbinding forces and kinetics of molecular complexes. Biophysical Journal, 94(7), 2621–2630.
Nishino, T., Matsui, R., & Nakamae, K. (1999). Elastic modulus of the crystalline regions of chitin and chitosan. Journal of Polymer Science Part B-Polymer Physics, 37(11), 1191–1196.
Ackbarow, T., Chen, X., Keten, S., & Buehler, M. J. (2007). Hierarchies, multiple energy barriers, and robustness govern the fracture mechanics of α-helical and β-sheet protein domains. PNAS, 104(42), 16410–16415.
Compton, O. C., Cranford, S. W., Putz, K. W., An, Z., Brinson, L. C., Buehler, M. J., & Nguyen, S. T. (2012). Tuning the mechanical properties of graphene oxide paper and its associated polymer nanocomposites by controlling cooperative intersheet hydrogen bonding. ACS Nano, 6(3), 2008–2019.
Keten, S., & Buehler, M. J. (2008). Geometric confinement governs the rupture strength of H-bond assemblies at a critical length scale. Nano Letters, 8(2), 743–748.
Acknowledgments
This work was supported by the National Natural Science Foundation of China through grant nos. 11222217, 11002079, and 31270989, Tsinghua University Initiative Scientific Research Program through grant nos. 2011Z02174 and 20121087991, and the Tsinghua National Laboratory for Information Science and Technology of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, K., Feng, X. & Xu, Z. Mechanical Properties of Chitin–Protein Interfaces: A Molecular Dynamics Study. BioNanoSci. 3, 312–320 (2013). https://doi.org/10.1007/s12668-013-0097-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-013-0097-2