Abstract
The Songwe geothermal prospect is situated in western Tanzania in the Rukwa Rift of the western branch of the East African Rift System. Thermal springs discharge along NW–SE oriented fracture zones in two separate areas: in the main Songwe graben (Iyola, Main springs, Rambo and Kaguri) and eastern Songwe graben (Ikumbi). Lithologies forming and filling the Songwe graben are metamorphic gneiss and shist as basement rocks, overlain by the Karoo sandstones, and Red sandstones, both silt- and sandstones with a carbonatic matrix. In some areas of the graben, volcanic rocks intruded these formations forming basalt outflows. The discharge temperatures of springs are between 37 and 85 °C with Na-HCO3 type fluids. Carbonate deposits surround most of the springs. Using previous geophysical, geological studies and historical fluid geochemical data and mineral data, the Songwe geothermal system interpretation was updated, including new reservoir fluid temperature, fluid flow pathway and water–rock interaction models. The classical geothermometers of K-Mg and Na-K-Ca (Mg correction) were used to predict the reservoir fluid temperature and show that fluid emerging in the Songwe area reaches temperatures between 125 and 148 °C. Reservoir fluid characteristics are reconstructed based on the geothermometer calculation and a PHREEQC model in which the deep fluid reacts with certain lithologies. Minerals precipitating at the surface and reservoir depth were used to calibrate the models. The models run at surface temperature were calibrated with minerals precipitating around the springs and suggest that Songwe thermal fluids interact with Red sandstone only, while Ikumbi spring water is the only spring that interacts with all lithologies (simplified referred to as: metamorphic rocks, Karoo and Red sandstone). The model run at reservoir temperature indicates that rising water is also in contact with Karoo sandstones and Ikumbi spring water composition is again influenced by the contact with all lithologies in the graben. Our conceptual model summarizes all data showing the meteoric origin of the fluids, the travel through the basement, rising along the Mbeya fault and the main reaction with sandstones through a lateral travel towards the hot springs. The proposed models reinforce the idea that carbonate dissolution from the sandstone layers is the most common water–rock interaction. Our model is supported by carbonate deposition observed in all springs, dominated by HCO3 and Na.
Similar content being viewed by others

Introduction
Geochemical investigations in the initial stage of geothermal field development typically aim for characterizing subsurface conditions to define drilling targets (Truesdell and Jones 1974). The geochemical information are obtained from geothermal surface manifestations. They do not only support temperature estimations but also give general information on the geothermal system such as hydrogeochemical processes, upflow, and/or outflow zones (Brehme et al., 2021; Nicholson 1993; White 1965, Ellis and Wilson 1960). Techniques usually applied are geochemical sampling and characterization of spring and reservoir water as well as mixing models to identify fluid sources, 2-phase proportions or mixing during fluid rise to surface (Truesdell and Jones 1974). Isotope studies help to identify the fluid source while geothermometers are used to estimate the reservoir temperature (Ellis and Wilson 1960). Equilibrium calculations of fluids with minerals are used to predict scaling tendencies (Giggenbach 1988a, b). In this study, the geochemical equilibration is used to identify the fluid source by comparing known precipitations to modelled fluid-rock interaction. By modelling the reaction of reservoir water with the different types of geological layers and comparing the results to measured minerals precipitated in that layer, we gain understanding of fluid pathways and sources.
Geochemical investigations related to geothermal energy exploration have been conducted in the Songwe field, Tanzania, since the late 1950s (see next paragraph). Located in western Tanzania in the Rukwa Rift of the western branch of the East African Rift System (EARS) the Songwe geothermal area has a significant resource with medium to high temperature thermal springs with carbonate or travertine-rich deposits (Hochstein et al. 2000). The system is located 50 km north of the Mbeya volcanic province.
James (1959) reported the first chemical analysis of thermal springs in the Songwe river valley. Using chemical data of the “Kaguri thermal spring” and gas samples of “Main hot springs”, the author suggests the gas-bearing springs are of volcanic origin. Nzaro (1970) also analyzed the thermal springs throughout the valley with a focus on their relation to the block-faulted region of the rift system. Later, a Swedish consultant group SWECO (1978) carried out a geochemical sampling survey of surface manifestations in Tanzania, including the Songwe area, and considered it one of the high-temperature geothermal fields in Tanzania. Makundi and Kifua (1985) provide a chemical data inventory of thermal springs in the Mbeya prospect area. Applying the Na-K-Ca geothermometer, these authors argue that the estimated deep fluid temperature is ranging from 170 °C near the Ivuna springs (80 km NW of Songwe) to 217 °C at Songwe. The other geothermometers of thermal springs (chalcedony, K/Mg, Na/K geothermometer) suggest intermediate to high temperatures (100–255 °C) in the Songwe reservoir although these springs are described as outflow zone (Hochstein et al. 2000; Mnjokava 2007). Springs in outflow zones normally show low temperatures in contrast to the upflow zone of a geothermal system.
These various datasets and the yet uncertain understanding of the geothermal system in Mbeya area led to the implementation of the GEOTHERM technical cooperation program of BGR (Federal Institute for Geoscience and Natural Resources, Germany) that consisted of a geological, geochemical, and geophysical survey between 2006 and 2009 (Delvaux et al. 2010; Kalberkamp et al. 2010; Kraml et al. 2010). Results show a structurally controlled fluid flow and a close association of thermal springs with active strike-slip and normal faulting (Delvaux et al. 2010). Magnetotelluric and Transient Electromagnetic surveys conducted by Kalberkamp et al. (2010) show a low magnetic anomaly in the Songwe area indicating strong alteration in rocks. Thus, the GEOTHERM studies came up with the main conclusion that Songwe thermal springs are an outflow zone of Lake Ngozi volcanic hydrothermal system. Conversely, other authors distinguish between Songwe geothermal system and Ngozi volcano based on the recent studies on geology, geochemistry, and geophysics by TGDC (Tanzania Geothermal Development Co. Ltd) and UNEP (United Nations Environment Programme) in 2016 (Alexander et al. 2016; Hinz et al. 2018). These authors also conclude that many springs are not associated with large apparent faults.
In addition, major ion analyses and mineral composition of travertines in the Songwe area have been studied to understand its origin showing a close association of the travertine deposits with Neogene-Quaternary volcanism (Pisarskii et al. 1998). Additionally, U/Th travertine data suggest a link between volcanic and geothermal activities (Delvaux et al. 2010). It supports the argument of Alexander et al. (2016) that Songwe Sr-rich travertines derive from volcanic rocks.
Even though a number of previous geoscientific studies have been carried out in the Songwe area, a detailed understanding of fluid flow pathways and related water–rock interaction has not been achieved yet. Taking advantage of the history of fluid geochemical data and geological mineral data of distinct lithologies, the purpose of this study is to update the interpretation of the Songwe geothermal system and add information on geothermal fluid pathways and sources. It comprises of (1) the reservoir temperature estimation and (2) interpretation of fluid flow pathways related to structural geology and (3) the modelling of water-rock interaction. We synthesize our results in a fluid flow model of the Songwe geothermal field.
Geology and structural geology
Regional geological setting
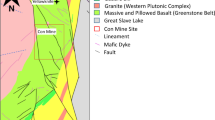
As part of the western branch of the EARS, Songwe geothermal resources are situated in the long valley of the Songwe basin. The Songwe basin is a sub-basin of the Rukwa Rift Basin, which is a half-graben flanked by uplifted Proterozoic metamorphic rocks of the late Ubendian shear belt (Kilembe and Rosendahl 1992). Metamorphic rocks in Songwe area are composed of amphibolite, gneiss, schist, and mylonite (EAGER 2018). The Proterozoic gneissic rocks consist of high-grade migmatitic metasediments (biotite-garnet gneisses) that are underlain by igneous rocks (hornblende-pyroxene gneisses and quartz-rich garnet pyroxene gneisses), and high-grade metamorphic rocks intruded by granodiorite and diorites (Harkin and Harpum 1978; Macfarlane 1966). These Proterozoic basement rocks (Fig. 1) are unconformably overlain by the Permo-Triassic Karoo group, Triassic Red sandstone, and Quaternary Volcanics (Fig. 1). In the western study area, the Permian-Triassic Karoo group is exposed at surface. These rocks are comprised of a series of glacial to periglacial strata, lacustrine, and fluvial deposits with conglomerates, siltstones and sandstone, carbonatic minerals and coal deposits (Alexander et al. 2016; Dypvik et al. 1990; Delvaux 2001; Roberts et al. 2004, 2010; Semkiwa et al. 1998). EAGER (2018) suggests the Karoo layers in the Songwe basin are mainly comprised of sandstone and conglomerate with about 500 m thickness.
Songwe geological map modified after Alexander et al. (2016), showing thermal spring locations
In the NW and the SE of the study area, the Red sandstone is widespread with 1300–1500 m thickness (EAGER 2018). The rocks consist of quartz, K-feldspar, muscovite and apatite (Alexander et al. 2016). The Red sandstones are loosely packed sediments, which are often overlain by massive travertine with 5–20 m thickness in the surrounding of thermal springs and extend towards the western basin margin (EAGER 2018). Additionally, fractures and pores in the Red sandstones are filled with carbonatic minerals. The Quaternary Volcanics are only deposited in the SE (Ikumbi) area and composed of trachyte and basalts (Alexander et al. 2016).
Structural geology of the Songwe basin
The geological cross section of the Songwe basin shows the basic geological units e.g. metamorphics as basement, overlain by Karoo Sandstones, Red Sandstones and partly volcanics (Fig. 2). The Mbeya fault in the NE of the basin cuts these units and underlines the graben structure. Sandstones and Volcanics are only found within the graben system and dip towards NE. Previous fluid-flow models suggest an infiltration of rainwater at the NE and SW edges of the basin, which is then heated at depth and rises through the sandstones to surface.
Schematic section across the Songwe half-graben. The overall geometry of the basin is obtained combining surface data from Alexander et al., (2016). The deep part of the basin is inferred from EAGER (2018). The two reports provide a contrasting hypothesis for the position of the boundary between the Karoo and Red Sandstones (dashed line in our figure). The flow paths correspond to those predicted by Alexander et al. (2016)
Associated with a rifting system, the Songwe basin is situated in an active structural set-up. The set-up has been investigated in several studies by (Alexander et al. 2016; Delvaux et al. 2010; EAGER 2018; Ebinger 1989; Hinz et al. 2018; Kilembe and Rosendahl 1992). All studies suggest a transtensional regime with strike-slip and normal faults. The main strike direction of the structures is corresponding to the basin in NW–SE direction.
Various studies differ slightly in their interpretation but show two major faults within the Songwe basin (Alexander et al. 2016; Delvaux et al. 2010; EAGER 2018). The Mbeya range fault is the NE boundary of the system and described as dextral strike slip or partly normal fault. Another major fault runs through the centre of the basin and is characterized as dextral strike-slip fault with a left step-over in the centre of the basin. This step-over zone is associated with fractures and accommodates most of the hot springs of the Songwe basin. Alexander et al. (2016) also suggested a fault at the SW boundary of the basin dipping as normal fault towards the basin. More recent studies by Hinz et al. (2018) additionally show fracture patterns and WNW striking, SW dipping normal faults within the basin. The detailed structural geology of the Songwe basin is subject of further studies by the authors of this paper and will be published in an additional format. Previous results confirm that the major dextral strike-slip fault in the basin centre provides locations of accompanying minor sinistral faults and a fracture pattern with normal displacement distributed over the basin.
Materials and methodology
Sampling and analysis
Water samples
A total of 17 geothermal springs from five spring groups were sampled during the first week of November 2019. The sampling locations are shown in Fig. 1. During the field survey, deposits around the thermal manifestations were described and physicochemical parameters were measured in-situ using portable instruments, including water temperature (T), acidity (pH), and specific electrical conductivity (EC). The sampling technique is applied according to hydrogeological procedures used by Brehme et al. (2010 and 2018). Water samples for chemical analysis were filtrated in-situ through 0.45 μm filter and stored in two 30 ml polyethylene bottles. Hydrochloric acid was added into one of the batches to lower the pH < 2 for cation analyses, while the other batch taken for anion analyses remained untreated.
The major ions were analyzed at the geochemical community laboratory of the Berlin Institute of Technology. Anion concentrations including Na+, K+, Fe2+/3+, Ca2+, and Mg2+ were determined by AAS (Analytik Jena, novAA 400) and ICP-OES Thermo Jarrel Ash with a detection limit of 0.02 ppm, 0.04 ppm, 0.0007 ppm, 0.007 ppm, and 0.003 ppm, respectively. Cations Cl-, SO42-, F-, Br-, NO3- and PO43-, were determined using ion chromatography Dionex DX 120 with detection limit of 1.5 ppm, 2.5 ppm, 0.25 ppm, 0,75 ppm, 0.75 ppm, and 1 ppm, respectively. The HCO3- concentration was measured on-site using alkalinity titration.
The reliability of the major element analysis was evaluated based on ionic balance errors. An ionic balance error is calculated by comparing the molal concentration of anions and cations (meq). An ionic balance error ≤ 5% means that the accuracy of the chemical analysis is reliable (Nicholson 1993). Practically, almost all data from the major elements analysis in 2019 are within the preferred ion balance error (Table 1) except for Kaguri 1 (6.6%).
Surface rock samples
Rock samples were taken at areas of emerging thermal springs. To identify mineral compounds, the surface rock samples were analyzed by X-ray diffraction (XRD) (Bruker D8 Advance diffractometer, with Cu Kα radiation and a Bragg-Brentano geometry) at the S-Rat Facilities Group of Delft University of Technology. About 1 gr of sample powder was deposited in PMMA holder L25, measured through XRD between coupled θ–2θ scan 10°–110°, with a step size 0.030° 2θ, and counting time per step of 1 s. The data interpretation has been done using Bruker software DiffracSuite.EVA versus 5.2. The porosity of the samples is measured using a He-Pycnometer ("Ultra Pycnometer 1000"). The permeability was measured using a portable handheld air permeameter from NER (https://www.ner.com/site/systems/tinyperm3.html). A rubber nozzle is pressed against the sample and withdraws air from it. The air is pulled from the sample and a microcontroller unit simultaneously monitors the syringe volume and the transient vacuum pulse created at the sample surface.
Geochemical modeling
To understand the geothermal fluid source in the Songwe basin we used geochemical modelling using PHREEQC version 3.6.2 (Parkhurst et al. 2013) with the Lawrence Livermore National Laboratory aqueous model database (llnl.dat) according to the scheme in Fig. 3. We tested possible thermal water interaction with all lithologies of the study area at the surface temperature and reservoir temperature. First, for the reservoir temperature model, the hot spring composition was calculated back to a reservoir fluid composition using the steam fraction produced by boiling at two reservoir temperatures (125 °C and 148 °C) (Fig. 3). In surface temperature models we first used measured chemical concentrations of the springs. In a second step, thermal water in springs and/or the calculated reservoir water react with different rock types of the study area to simulate water-rock-interaction in this specific lithology at different temperatures. This is done to test if the resulting fluid composition and precipitating minerals match with the measured values. The reaction in the equilibrium model gives saturation indices (SI) for different minerals. A negative SI suggests dissolution of the mineral while a positive SI suggests precipitation of the mineral (Brehme et al. 2016). Modelled precipitated minerals in the surface temperature were compared to observed precipitated minerals at the hot springs. In the reservoir temperature model, modelled precipitated minerals were compared to measured minerals in the rocks. The composition of the rock was taken from XRD data and literature (Table 2). The best match indicates the real water-rock interaction and marks the lithology where the geothermal fluid originates. Thus, the reaction model improves our understanding of the water-rock interaction in the geothermal system and indicates the geothermal fluid source in the Songwe basin.
Results
Thermal spring characterization
Songwe geothermal springs can be divided into five groups, based on their location: Iyola, Main springs, Kaguri, Rambo, and Ikumbi. Except Ikumbi springs, they are located along the Songwe river, at the west side of the Songwe graben. The graben represents a NW-SE extended structure with a river and carbonate deposits surrounding the springs (Fig. 1). Iyola springs (62 to 74 °C) rise near to the river and from the river bed below the water with gas bubbles (Table 1). SW of Iyola and 50 m elevated, Main springs discharge from thick-blocky travertine rocks with temperatures of about 68 to 75 °C. In the western-central part of the Songwe graben, Rambo springs discharge with higher temperatures ranging between 70 and 83 °C also from travertine rocks. Kaguri springs discharge close to Rambo but at a lower elevation in the river bed with lower temperatures of 48 to 71 °C. Approximately ~6 km southeast of Rambo springs along the Ikumbi river, Ikumbi springs rise, with the lowest temperature of 37 to 44 °C in the study area. The most abundant rocks at Ikumbi springs are volcanic rocks.
The pH of the thermal springs is near neutral to slightly alkaline (5.8–8.3), except for ‘Rambo new’ spring which exhibits a slightly acidic pH (4.8) (Arnorsson et al. 1982; Reed and Nicolas 1984; Truesdell and Jones 1974; Wilson 1961). The electrical conductivity (EC) ranges from 410 to 3600 μS/cm in our measurements and 3830 μS/cm in former studies. The highest EC occurs in the Iyola springs (in former studies in Main springs) with increased concentrations of all common ions, while Ikumbi springs show the lowest EC (Table 1).
The dominant anion in Songwe geothermal springs is HCO3, ranging from 430 to 2248 mg/L in our measurements compared to up to 3580 mg/L in former studies. Also, the Cl-SO4-HCO3 triangular diagram (Fig. 4) shows, that all the springs plot in the HCO3 apex. Interestingly, Ikumbi springs show high concentrations of SO4 and Cl but contain the lowest absolute bicarbonate. Cl and SO4 are the second most abundant anions with lower concentrations of 8 to 223 mg/L, and 5 to 178 mg/L (in former studies up to 227 and 430 mg/L), respectively. Cl concentrations appear within the range of common geothermal fluids of about <10 to 100,000 mg/kg (Nicholson 1993). The most dominant cations in the springs are Na with up to 980 mg/L, and K, Ca, Mg, Li and Fe, with lower concentration of 44–107 mg/L (formerly up to 114 mg/L), 12–61 mg/L (formerly 10–49 mg/L), 5–24 mg/L (formerly 3–20 mg/L), 0.02–0.79 mg/L, and less than 0.02 mg/L (formerly up to 0.17 mg/L), respectively (Table 1). The low concentrations of these cations are common for geothermal fluids. In high temperature fluids, Ca and Mg typically occur at low levels of about < ~50 mg/kg and 0.01–0.1 mg/kg, respectively. (Nicholson 1993).
Rock composition
All geological layers have been observed during field work in 2019 mostly in unaltered conditions. XRD analysis show typical sandstone minerals for Karoo and Red Sandstone layers, e.g. quartz, feldspar and muscovite. Several minerals have been detected in the Karoo Sandstone as indicators for high-temperature fluid-rock interaction, e.g. illite, smectite and kaolinite (Table 1). Fractures, observed especially in Red Sandstone and Karoo Sandstone outcrops, were filled with carbonate minerals. They form ~20% of the rock volume. Effective porosity and specific permeability measured in the laboratory show low values of maximum 21% porosity for the Red Sandstones and a maximum permeability of 1.3 x10-12 m2 for Karoo Sandstones (Table 2). Geothermal manifestations mostly arise from travertines or Red Sandstone rocks, while Ikumbi springs are located within the Quaternary Volcanics in the east.
Element ratios in thermal waters
The relative concentrations of lithium, chlorite and boron of Songwe thermal springs are shown in Fig. 5. Most of Songwe thermal springs plot close to the Cl corner, near the Cl-Li line and some close to older hydrothermal systems. Ikumbi springs show a slightly higher Cl relative to Li and B than other springs. All the springs plotting in Fig. 5 are consistent with the results of previous studies, except Rambo and Main springs in 2007, which show a slight increase in B content.
The Cl/B ratios show different trends for Ikumbi springs (89–616) compared to the other Songwe springs (103–111). Ikumbi springs also have a higher Cl/As ratio compared to the other springs. Our study shows that Kaguri and Rambo springs have high Na/Mg (>100) and Cl/Mg ratios (>14), a similar value to that presented in previous studies (Table 3).
Geothermometry
Solute geothermometers use variations of element concentrations in hot springs as a function of temperature to calculate the temperature of a geothermal system. Geothermometers are based on several assumptions including full equilibration and no-mixing, which not always meet realistic conditions (Fournier 1977; Nicholson 1993). HCO3-waters, for instance, are often considered to occur far from the reservoir as a result of steam heating and condensation. However, depending on the geological setting, they can also represent deep reservoir fluids in non-volcanic but high temperature systems such as in Turkey and Africa (Nicholson 1993). Therefore, the solute geothermometer calculations are still applied for Kaguri and Rambo springs, which have high surface temperatures, and high HCO3 concentrations. Additionally, the Na-K-Mg ternary diagram is used to estimate reservoir temperatures and shows that Kaguri and Rambo springs are partially equilibrated waters, which allows using them for geothermometer calculations (Fig. 6).
In the Na-K-Mg diagram, Rambo shows a distinctive span of Na-K temperatures over the years (Fig. 6). Some samples plot in the range of 200 to 240 °C, exemplary for high-temperature systems, and some at 120 to 150 °C exemplary for low temperature systems. To clarify the appropriate Na-K temperature for the study area, the Na-K geothermometer proposed by Fournier (1977) and Giggenbach (1988a, b) is used. The results show that Kaguri and Rambo springs equilibrated at a subsurface temperature range of 216 to 232 °C (Table 4). Slightly higher temperatures have been proposed in previous studies: 217 to 256 °C for Rambo springs, 245 and 259 °C for Kaguri springs. The Na-K-Ca geothermometer also shows high temperatures of >200 °C (Table 3). A Mg correction for the Na-K-Ca geothermometer, because Mg is very high in the Songwe thermal springs (up to 18 mg/L), then gives lower temperatures of 116 to 142 °C. Likewise, the K-Mg geothermometer suggests lower temperatures (125–148 °C) assuming interfering fast kinetic reactions with K and Mg, thus a faster re-equilibrium of the fluid. The low temperatures estimated by previous studies is confirmed by SiO2 quartz geothermometry results, which predict that Songwe fluids equilibrated at 113 to 128 °C (Table 3). Although the chalcedony geothermometer predicts even lower temperatures of 82 to 104 °C, the correlation of SiO2 content and C2K/CMg indicates that mostly Kaguri and Rambo thermal waters are in equilibrium with SiO2 quartz (Fig. 7).
Diagram of SiO2 versus log K2/Mg of Songwe thermal water, concentration in mg/l, based on Giggenbach and Soto (1992). The curves provide information on equilibrium attainment for SiO2 and K-Mg geothermometers. Most thermal water plots near the quartz curve, except for Rambo (1978) which plots between quartz and chalcedony curve, suggesting that equilibria with quartz took place rather than with chalcedony
Mixing model
Geothermometer application for mixed thermal waters can influence the reliability of the estimated temperature. Thus, two mixing models were applied to evaluate the subsurface temperature of the Songwe area, estimated by solute geothermometers. Figure 8 shows the silica-enthalpy model (Truesdell and Jones 1974) for Rambo, Main springs, Kaguri and Ikumbi thermal springs. The mixing line is based on the sample with the highest and lowest silica content. The estimated temperature of the mixed thermal springs is between 100 °C and 124.5 °C based on the silica (chalcedony)-enthalpy mixing model, but the silica (quartz)-enthalpy mixing model shows higher temperature between 128.5 °C and 148.3 °C. The chlorite-enthalpy mixing model results in a temperature of 142.5 °C. Additionally, another evidence for mixing processes in our geothermal waters is shown by the relationship between Cl and conservative species B in Fig. 9. All the thermal springs show a linear relationship between Cl and B.
Mixing model of silica-enthalpy (based on chalcedony and quartz geothermometry temperature) and enthalpy-chloride (based on quartz geothermometry temperature). Silica concentration from previous geochemistry studies (see Table 1). The mixing lines on both diagrams are drawn from cold Songwe river water to the quartz solubility and to the steam line. Intersection points are based on the spring with higher concentration of silica (line A) and lower concentration (line B). Ikumbi springs were excluded from the mixing line because they were suspected as steam-heated waters
Reservoir fluid chemistry
The fluid characteristics for the reservoir temperature were reconstructed using one representative spring water composition out of each group: Rambo old, Kaguri 1, Main spring 2, Iyola 1 and Ikumbi 4. Concentrations of all species were calculated for 125 °C and 148 °C, representing the Songwe deep reservoir temperature, based on K-Mg geothermometer results. At these temperatures, it is assumed that the steam separates from the fluid phase during boiling and ascent to the surface. After the steam fraction is subtracted from the reservoir fluid, the liquid phase has a smaller mass and increased concentration of elements. Therefore, all solute concentrations of the reservoir fluid in Table 5 are lower than thermal springs at surface.
The pH of the reservoir fluid can be estimated using mineral equilibria reaction. Carbonate and especially calcite dissolution equilibria are commonly used to get a close approximation of the reservoir pH. First, carbonate equilibria are used in this study since Songwe thermal springs are dominated by bicarbonate evolving from the dissociation of dissolved carbon dioxide into bicarbonate and hydrogen in equilibrium governing described (Nicholson 1993):
The XRD data of rock samples in all springs, also show calcite minerals, thus, we additionally applied the calcite dissolution equilibria. The calcite method gives a lower pH value (5.8–6.3) than the carbonate method (6.0–6.2, Table 5). The differences in pH from both methods are the consequence of carbonic species concentration in the sample. The pH also differs depending on temperature. For the calcite equilibria, the lower pH (5.8) occurs at 148 °C which increases with decreasing temperature (125 °C: 6.3). The bicarbonate method has a reverse trend. Based on the assumption that pH varies with temperature and a neutral pH 7 observed at surface, a pH of ~ 5.5 at higher reservoir temperatures at depth is expected (Nicholson 1993). In our case, the pH calculation with calcite dissolution equilibria shows more reliable results.
Mineral saturation indices
To calculate mineral saturation indices, the Phreeqc program (Parkhurst and Appelo, 2013) is used to identify possibly precipitating minerals, shown by positive saturation indices. Our model represents thermal water at surface temperature and at reservoir temperature reacting with different lithologies in the study area. Input data are thermal spring compositions and calculated reservoir fluid compositions at temperatures of 125 °C and 148 °C including pH, Na, K, Ca, Mg, Fe, HCO3, SO4, and Cl (Tables 1 and 5). The primary minerals of each lithology in the Songwe area are from XRD data of rock samples and literature (Table 2).
There are eight scenarios modelled for the hot spring cluster at surface conditions: scenario I-V for Rambo, Kaguri, Main springs, and Iyola, and scenario VI, VII and VIII for Ikumbi springs, including volcanics observed at shallow depth (Table 6). At reservoir conditions, scenario I-V were modelled for reservoir fluids reconstructed from all springs, but scenario VI-VIII for Ikumbi springs only. In scenario I and IV, the thermal water reacts with a single lithology (Metamorphic rocks or Red Sandstone). While in scenario II, III, V, VI, VII and VIII, a mixture of lithologies from oldest to youngest (Metamorphic, Karoo, Red Sandstone, Volcanic) reacts with the water simultaneously. The reaction delivers a new fluid composition and oversaturated minerals, which precipitate at reservoir depth. The results are then compared to sampled water and mineral compositions as calibration data, to test which rocks the reservoir water has been in contact with. The resulting oversaturated minerals were divided into groups: weathered volcanics, clays, apatites, weathered metamorphics, and hydrothermal minerals. Their exact saturation indices and abundance vary per scenario (Supplementary Material: Table S1a-e and S2a-h). In the surface temperature model, the modelled oversaturated minerals are compared to minerals encountered at the hot springs and the real hot spring water composition to understand reservoir water flow pathways and its interaction with rocks. In more detail, minerals precipitating at hot springs derive from dissolved solids in the reservoir water, attained through water-rock interaction at depth. Our model can show at which rock layer the finally precipitating minerals have been dissolved.
Surface temperature model
The output of the reaction between thermal springs at their surface temperature with Metamorphics (scenario I) is dominated by weathered metamorphics with up to 19.5 SI in Kaguri, while the lowest SI is observed in Main springs (Supplementary Material: Table S1a). The second-highest SI values appear for apatites, while the lowest appear for carbonates and only in Kaguri (SI ~ 1).
Scenario II involves the reaction between water, Metamorphic and Karoo rocks and shows that Rambo shows the highest SIs in all mineral groups, but no spring shows a supersaturation of carbonates (Supplementary Material: Table S1.b). SIs of around 1 to 3 are observed for clays and weathered metamorphics in all other springs. Apatite, clays and metamorphics show especially high SIs of 12-18 in Rambo.
In scenario III, clays show again the highest variety of supersaturated minerals (Supplementary Material: Table S1.c). Weathered metamorphics show the highest SIs of 10-12. Also, hydrothermal minerals show high SIs of 11-12, besides in Kaguri. Only Kaguri and Main springs show carbonates as supersaturated.
Scenario IV model results (Supplementary Material: Table S1.d) show clay minerals with the highest variety and highest SIs of supersaturated minerals for the reaction of water with Red Sandstone. The second highest SIs occur for weathered metamorphics in all springs. All springs also show a supersaturation of carbonates, especially for the first time all thermal spring waters are supersaturated with aragonite and calcites.
The reaction of thermal waters with all lithologies in scenario V (Supplementary Material: Table S1.e) shows similar SIs for all springs. Clays are still the minerals with the highest variety. Highest SIs occur for weathered metamorphics and hydrothermal minerals. Carbonates show the lowest SIs and do not precipitate in Rambo springs.
The reaction between all lithologies and thermal water is only modelled for Ikumbi (scenario VI, Supplementary Material: Table S1.f). Highest SIs of 10-15 appear for clays, carbonates, weathered metamorphics and hydrothermal minerals. The lowest SIs occur for clays. Muscovite is observed to be supersaturated and also observed at surface close to the spring.
Reservoir temperature model
The model results show that in scenario I (Supplementary Material: Table S2.a), most supersaturated minerals are weathered metamorphics, followed by clays and weathered volcanics. For weathered metamorphics and clays the SIs tend to be higher at 148 °C, while they tend to be lower for weathered volcanics at higher temperatures. The highest SIs appear for weathered metamorphics with several values of 10–20. Clays show SIs of ~12 and weathered volcanics mostly of 1–8. The water-rock reactions in scenario I involve metamorphic rocks as the only input, a possible reason for the high abundance of weathered metamorphics in the model outcome.
Scenario II model results (Supplementary Material: Table S2.b) show the highest variety in supersaturated clay minerals. However, their SIs are mostly around 1 with increased values at higher temperatures. The highest SIs of 4–11 occur for apatites, that are also observed at the hot springs. Weathered volcanics precipitate at all springs beside Rambo, while weathered metamorphics are supersaturated at all springs. These different trends between the springs, are due to a higher variety in mineral composition in scenario II, representing metamorphics and Karoo.
The reactions at 125 and 148 °C in scenario III also show the clay minerals group as the most varying minerals with SIs around 8 (Supplementary Material: Table S2.c). The least supersaturated minerals are weathered volcanics and carbonates. Apatite and hydrothermal minerals show high SIs of 6-15 and is also found as surface mineral at the springs.
In scenario IV, clay minerals are still showing the highest variety and SIs of 2–12 with the highest values at Ikumbi (Supplementary Material: Table S2.d). The SIs decrease with increasing temperature. Carbonates are supersaturated in all springs with SIs around 1–3 and especially Calcite is also found as surface mineral at the springs. Apatites show higher SIs of 5–13 with increasing supersaturation at increasing temperature. Weathered metamorphics and volcanics are less abundant.
In accordance with other scenarios, scenario V shows at both temperatures of 125 °C and 148 °C, clays minerals as the varying mineral group with SIs of 1–10 (Supplementary Material: Table S2.e). Smectite is supersaturated in Rambo, Main springs, Iyola and Ikumbi (125 °C). Carbonates precipitate mainly in Kaguri, and a little bit in Iyola and Ikumbi springs. Calcite has also been observed onsite at the hot spring locations. Apatites are supersaturated in all springs with SIs of 6–19 and also observed as minerals at the locations. Weathered metamorphics, volcanics and hydrothermal minerals occur at single hot springs with lower SIs of 1–8 mainly. Clays show higher SIs, while carbonates and apatites show lower SIs at increased temperature. The enrichment of clay minerals is also visible in decreasing K in the thermal springs in 2019 and the K uptake in secondary clays seen in the K-Na-Mg-Ca diagram (Fig. 10).
The plot of weight-fractions of 10 K/(10 K + Na) and 10 Mg/(10 Mg + Ca) adapted from Giggenbach (1988a), b for thermal waters in Songwe. The diagram represents also general processes including water equilibrium with average crustal rocks along the “full equilibrium” line; “rock dissolution” as isochemical dissolution of crustal rocks (Giggenbach 1988a, b); and water mixing with seawater. None of Songwe thermal springs plot close to full equilibrium line
Scenario VI, VII and VIII results show a strong supersaturation of weathered volcanics at both 125 °C and 148 °C (Supplementary Material: Table S2.f, S2.g, and S2.h). In scenario VI also clays and apatites are supersaturated as well as found at the thermal springs. Scenario VII and VIII show less minerals supersaturated but high SIs of 10-18 for weathered volcanics and apatites. Lower SIs of 1-6 are found for clay minerals.
Discussion
Hydrogeochemistry
The results show that all the thermal springs in the Songwe area have a Na-HCO3 water type. This is related to the presence of bicarbonate in the reservoir rocks. At Ikumbi springs located within volcanic rocks, the HCO3 can also form near surface when CO2 gas and steam condenses into cold water (steam heated water). The enthalpy-chloride model also shows that Ikumbi thermal waters plot in the steam heated water area (Fig. 8).
Kaguri, Rambo, Main springs, Iyola and Ikumbi have a similar water type, but different fluid pathways or a change of lithology at depth, shown by different element ratios. Element ratios can be used to identify the common source of reservoir fluids and identify physicochemical processes in a geothermal system (Nicholson 1993). Relatively high Na/Mg and Cl/Mg ratios are typical for high temperature fluids, that reach the surface rapidly defining the upflow zone of the system (Nicholson 1993). The ratios suggest that Kaguri and Rambo are situated in the upflow zone and are directly fed from the reservoir. This is in good agreement with high surface temperatures observed in both springs. Thus, Songwe thermal springs form a geothermal area with an independent geothermal (heat) source and are not an outflow of the Ngozi volcanic geothermal system as suggested by previous studies (GEOTHERM; Delvaux et al. 2010). During their rise to the surface, there is a chance that Songwe springs might mix with cold groundwater in the upflow zone as seen in Fig. 9 that shows a linear relationship between Cl and B. As a conservative species in geothermal fluids, Cl content will increase continuously during progressive water–rock interaction.
The alkali metal Li, which is least affected by secondary absorption processes, is typically used together with conservative elements B and Cl for evaluating a possible origin of geothermal fluids (Giggenbach and Soto 1992). Our results suggest that Songwe thermal springs were formed through absorption processes with low B/Cl magmatic vapor input and originate from an older hydrothermal system. Tritium data (Kraml et al. 2008) have been used to explain that Songwe thermal springs have very low (0.00 ± 0.02) tritium, suggesting relatively high residence times in the subsurface.
Reservoir temperature estimation
Classical geothermometers have been applied to Songwe geothermal springs which yield a wide range of temperatures from previous geochemistry studies. Makundi and Kifua (1985) argued that as part of the Mbeya volcanic area, Songwe is a high enthalpy system with temperatures of 217 °C based on the Na–K-Ca geothermometer. However, the Na–K-Ca geothermometer shows unreliable high temperatures because it is applied to HCO3 waters in a low temperature system (Pope et al., 1987). Likewise, this study shows overestimated Na–K geothermometer results because of Kaguri and Rambo springs not being fully equilibrated and kinetic reactions including Na and K causing a slow re-equilibration (Fig. 6; Giggenbach 1988a, b; Hochstein 1988). In contrast, this study suggests that Songwe is a low to medium enthalpy geothermal system. K-Mg geothermometers and Na–K-Ca(Mg correction) (Table 4) give reasonable approximations of reservoir temperatures in the range of 125 to 148 °C. This is in good agreement with Alexander et al. (2016) who also predicted lower temperatures (112 ± 16 °C) based on K-Mg, Silica and quartz geothermometers.
The silica-enthalpy and enthalpy-chloride mixing models show that the temperature of possible parent fluid in the study area are in the range of 100 to 149.5 °C. Those numbers are higher than those obtained by the quartz geothermometer (Fig. 8 and Table 3), which suggests that most of the hot water is mixing with cooler water from shallow aquifers and/or that conductive cooling takes place during the ascent of the geothermal fluid to the surface.
Water–rock interaction modeling
Each of our hydrogeochemical models results in possibly precipitating mineral groups, while percentage and composition differ. A comparison with the mineral composition of the reservoir rocks and minerals precipitated at surface we obtained best fits of our models. The best fitting scenarios are summarized in Fig. 11, including saturation indices and percentages. Lithologies are shown in coloured layers including porosity and permeability values. Arrows show the reaction pathway, covering reactions with 1–3 layers and resulting saturation indices. The highlighted minerals in blue and yellow correlate with the observed minerals at each thermal spring. Figure 11a focuses on carbonate precipitation, Fig. 11b also shows other precipitating minerals at surface temperature. At Rambo, Kaguri, Iyola, and Main springs only carbonate minerals were detected at surface, while at Ikumbi mainly analcime, muscovite, and smectite have been detected. The best fitting model, showing carbonate precipitation in the model and in real case at Rambo, Iyola, Kaguri, and Main springs is when the fluid reacts only with Red sandstone (scenario IV). For Ikumbi, scenario VI (reaction with Metamorphics, Karoo, Red Sandstone and Volcanics) results in the best fit. Figure 11b presents the final results of the model, showing that Iyola, Main springs, Kaguri and Rambo spring water is mainly dissolving minerals from the Red Sandstone, resulting in massive carbonate precipitation at surface. Also, Ikumbi waters have been in contact with all lithologies, resulting in the precipitation of Analcime, Chabazie-Ca, Muscovite, and Smectite. Figure 11c, d show the reaction results at reservoir condition. The precipitation of measured minerals is only found in modelled reactions of scenario II, III and V for Iyola, Main springs, Kaguri and Rambo and scenario III, IV, VI, VII and VIII for Ikumbi. The best fitting model for Rambo, Iyola, Kaguri and Main springs at 125 °C and 148 °C is when the reaction involves the fluid, Karoo and Red sandstone (scenario III). It shows most secondary minerals precipitating (group of apatites, clays and hydrothermal minerals) in the modelled reaction and the measured minerals. Scenario VI is the best fitting model for Ikumbi where the group of apatites, clays and weathered volcanics precipitate in the modelled reaction confirmed by measured data. This is consistent with the Li-Cl-B data of Ikumbi (Fig. 5) that show a slightly higher Cl content relative to Li and B in other springs indicating that Ikumbi migrated from fluid in the old basement rock.
The scheme of water–rock interaction for surface thermal springs (a and b) and for estimated reservoir water (c and d) at 125 °C (black ink) and 148 °C (white ink) when reacting with different lithology in the Songwe geothermal area. Lithologies are shown in different colors including porosity and permeability values. Arrows show the reaction pathway, covering the reaction with 1–3 layers and resulting saturation indices. The highlighted minerals in blue correlate with the highlighted minerals at surface of each thermal spring. a model outputs only focusing on calcite (calc.) and aragonite (arag.); it suggests the thermal springs are only in contact with Red Sandstone, and Ikumbi is also in contact with metamorphic rocks, Karoo group (longest arrow) and younger volcanics. Ikumbi springs react with all lithologies in the Songwe field, supported by the precipitation of analcime, muscovite and smectite. b model outputs including all mineral groups: Apt (apatite), Car (carbonates), Clay, WM (weathered metamorphics), and WV (weathered volcanics). c model outputs focusing on minerals measured and precipitated in the model reactions consisting of HAp (Hydroxyapatite), FAp (Flouroapatite), Illite, Smec (smectite). Cl (chlorite), Non-Ca (nontronite-Ca), Non-Na (nontronite-Na), Cal (calcite) and Kao (Kaolinite). d model outputs of mineral groups percentage for the best fitting scenario. aThis study; bBeyer and Clutsom (1978)
Conceptual fluid flow model
Integrating all data from this and former studies are summarized in a conceptual fluid flow model for the Songwe basin. Generally, faults and fractures control the fluid flow in the regional setting of the NW trending basin. Recharge happens most probably through the higher elevations NE of the Mbeya fault (Alexander et al. 2016). Meteoric water infiltrates here and mixes with deep hot fluids originating from the 40-km thick crust beneath the Songwe basin Kraml et al. (2008). This water is stored mainly in the metamorphic basement and ascends through faults and fractures in the basin. Water-rock interaction during the rise leads to secondary hydrogeochemical processes that change the chemical composition of thermal water.
Both models suggest that during ascension, fluids reach more permeable lithologies like the Karoo and Red Sandstones. Due to higher permeability fluids now also percolate laterally within the sandstones and have a longer reaction time with these carbonate rich rocks. They eventually rise towards the surface at minor fracture sets, as observed at Rambo, Kaguri, Main springs and Iyola. Ikumbi water additionally is in contact with volcanic rocks overlaying the sandstones. Both hydrochemical models, calibrated with reservoir or surface temperature show, that these fluids are dominated by water-rock-interaction in the Red sandstone (Fig. 12).
Conceptual fluid flow model of the Songwe area after hydrogeochemical model interpretation. Showing fluid infiltration in the NE and supposedly SW, fluids rising along the fault and percolating within the sandstones before discharging in hot springs (Christopher 2015)
During ascent of the fluids, temperature and pressure drops, resulting in cooling and degassing of CO2. Degassing results in decreased solubility of HCO3 and calcite/aragonite oversaturation, causing massive travertine deposits. High HCO3 and Na concentrations in all spring waters support the theory of carbonate dissolution from the Red sandstone layer as the most common water-rock interaction in the Songwe basin.
Conventional geothermometry predicts reservoir temperatures in the range of 125 to 148 °C. The hydrochemical model at reservoir condition shows that thermal fluids react with Karoo and Red sandstone in the study area. The higher the reservoir temperature, the deeper the lithology in contact with thermal fluids.
Conclusion
This study presents a fluid source model of the Songwe geothermal system using data on the composition of thermal spring fluids, primary minerals of lithologies, and a water–rock reaction model. The medium to high temperature thermal waters of neutral pH and Na-HCO3 type are driven by advective flow through interconnected fault/fracture networks. Fluid-rock interaction within permeable zones causes re-equilibrium of fluids with rocks, in fast kinetic reactions of mainly Mg with K, as well as Na and Ca with Mg correction. The K-Mg and Na–K-Ca (Mg correction) geothermometer shows an equilibrium temperature for the reservoir fluid between 125 and 148 °C. The reconstruction of the chemical fluid composition under reservoir condition and at surface temperatures are used to identify water–rock reaction scenarios most suitable for mimicking measured minerals in the rocks and for imitating minerals precipitating at the surface (i.e. calcite, aragonite, analcime, muscovite, and smectite). There are three best fitting reaction scenarios for fluid-rock interaction for different conditions and it can be used to identify the thermal water source: scenario IV for Kaguri, Rambo, Iyola, and Main springs, at surface temperature and scenario III at reservoir temperature and scenario VI for Ikumbi springs at both conditions. The thermal springs have been in contact with Red sandstone only at surface temperature, but at the higher temperatures, thermal water also reacts with deeper lithologies like Karoo and Red sandstone. Ikumbi is in contact with all lithologies including Younger Volcanics. These results are supported by relatively high Na/Mg and Cl/Mg ratios at Rambo and Kaguri, as well as partial equilibration of thermal spring water, which suggests that Rambo and Kaguri are directly fed from the reservoir and located in the upflow zone of the Songwe geothermal system. The other springs are located at the outflow zone, which is generally controlled by NW–SE trending faults and fractures.
References
Alexander K, Cumming W, Marini L (2016) Geothermal resource assessment report: Ngozi and Songwe Geothermal Prospects, Tanzania, Final Report dated September 2016 to UNEP/ARGeo and TGDC.
Arnorsson S, Sigurdsson S, Svavarsson H (1982) The chemistry of geothermal waterS in Iceland. I. Calculation of aqueous speciation from 0° to 370°C. Geochim Cosmochim Acta 46:1513–1532
Beyer LA, Clutsom FG (1978) Density and porosity of oil reservoirs and overlying formations from borehole gravity measurements Gebo Oil Field, Hot Springs County, Wyoming. Oil Gas Invest Chart. https://doi.org/10.3133/oc88
Brehme M, Scheytt T, Çelik M, Dokuz UE (2010) Hydrochemical characterisation of ground and surface water at Dörtyol/Hatay/Turkey. Environ Earth Sci 63:1395–1408. https://doi.org/10.1007/s12665-010-0810-1
Brehme M, Deon F, Haase C, Wiegand B, Kamah Y, Sauter M, Regenspurg S (2016) Fault controlled geochemical properties in Lahendong geothermal reservoir Indonesia. Grundwasser 21:29–41. https://doi.org/10.1007/s00767-015-0313-9
Brehme M, Regenspurg S, Leary P, Bulut F, Milsch H, Petrauskas S, Valickas R, Blöcher G (2018) Injection-triggered occlusion of flow pathways in geothermal operations. Geofluids 2018:4694829. https://doi.org/10.1155/2018/4694829
Brehme M, Giese R, Dokuz UE, Bulut F (2021) Fluid pathways identified beneath Narlı Lake (Central Anatolia) show the geothermal potential of former volcanoes. Sci Rep 11(1):1–8
Christopher B (2015) Geological and geophysical investigation of the southeastern Karoo basin. University of Fort Hare, South Africa, South Africa
Delvaux D, Kraml M, Sierralta M, Wittenberg A, Mayalla JW, Kabaka K, Makene C (2010) surface exploration of a viable geothermal resource in Mbeya Area, Sw Tanzania. Part I: geology of the Ngozi-Songwe geothermal system. Proc. World Geotherm. Congr. 25–29.
Delvaux D (2001) Karoo rifting in western Tanzania: precursor of Gondwana break-up ? Contrib. to Geol. Palaeontol. Gondwana honour Helmut Wopfner 111–125.
Dypvik H, Nesteby H, Ruden F, Aagaard P, Johansson T, Msindai J, Massay C (1990) Upper Paleozoic and Pesozoic sedimentation in the Rukwa-Tukuyu Region Tanzania. J African Earth Sci 11:437–456. https://doi.org/10.1016/0899-5362(90)90022-7
EAGER, 2018. Enhanced resource modeling at Songwe.
Ebinger CJ (1989) Geological society of America Bulletin Tectonic development of the western branch of the East African rift system. Geol Soc Am Bull 7606:885–903. https://doi.org/10.1130/0016-7606(1989)101%3c0885
Ellis AJ, Wilson SH (1960) The geochemistry of alkali metal ions in the Wairakei hydrothermal system. New Zeal J Geol Geophys 3:593–617. https://doi.org/10.1080/00288306.1960.10420148
Fournier RO (1977) Chemical geothermometers and mixing models for geothermal systems. Geothermics 5:41–50. https://doi.org/10.1016/0375-6505(77)90007-4
Giggenbach WF (1988a) Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim Cosmochim Acta 52:2749–2765. https://doi.org/10.1016/0016-7037(88)90143-3
Giggenbach WF, Soto RC (1992) Isotopic and chemical composition of water and steam discharges from volcanic-magmatic-hydrothermal systems of the Guanacaste Geothermal Province. Costa Rica Appl Geochem 7:309–332. https://doi.org/10.1016/0883-2927(92)90022-U
Giggenbach WF (1988b) Geothermal solute equilibrium, derivation of the Na−K−Mg Geoindicator.pdf. https://doi.org/10.1016/0016-7037(88)90143-3
Harkin D, Harpum J (1978) Geological Map of Tukuyu (QDS 244), in: Tech. Rep. Geological Survey of Tanganyika, Dodoma.
Henley RW, Ellis AJ (1983) Geothermal systems ancient and modern : a geochemical review 19.
Hinz N, Cumming B, Sussman D (2018) Exploration of fault-related deep-circulation geothermal resources in the western branch of the East African Rift System: examples from Uganda and Tanzania. Proc. 7th African Rift Geotherm. Conf. 2018. Kigali, Rwanda. 16 p.
Hochstein MP (1988) Assessment and modelling of geothermal reservoirs (small utilization schemes). Geothermics 17:15–49. https://doi.org/10.1016/0375-6505(88)90004-1
Hochstein M, Temu E, Moshy CM (2000) Geothermal resources of Tanzania. Proc World Geotherm Congr Japan. https://doi.org/10.1016/0375-6505(70)90412-8
James C (1959) Carbon-dioxide-bearing hot springs in the Songwe River Valley, Mbeya District. Tanganyika Geol Surv Rec 7
Kalberkamp U, Schaumann G, Ndonde PB, Chiragwile AS, Mwano MJ, GEOTHERM, working group (2010) Surface Exploration of a Viable Geothermal Resource in Mbeya Area, SW Tanzania. Part III: Geophysics Ulrich. Proc. World Geotherm. Congr.
Kilembe EA, Rosendahl BR (1992) Structure and stratigraphy of the Rukwa rift. Tectonophysics 209:143–158. https://doi.org/10.1016/0040-1951(92)90016-Y
Kraml M, Schaumann G, Kalberkamp U, Stadtler C, Delvaux D, Ndonde P, Mnjokava T, Chiragwile S, Mayalla J, Kabaka K, Mwano J, Makene C (2008) Geothermal Energy as an Alternative Source of Energy for Tanzania, Final Technical Report of Phase I (2006–2009). Technical Cooperation with United Republic of Tanzania
Kraml M, Mnjokava TT, Mayalla WJ, Kabaka K, GEOTHERM G. working (2010) Surface Exploration of a Viable Geothermal Resource in Mbeya Area, SW Tanzania Part II: Geochemistry, In: Proceedings World Geothermal Congress.
Macfarlane A (1966) Geological Map of Itumba (QDS 258), In: Tech. Rep. Mineral Resources Division, Dodoma.
Makundi JS, Kifua GM (1985) Geothermal features of the Mbeya prospect in Tanzania. Geotherm Resour Counc 9:451–454
Minissale A, Donato A, Procesi M, Pizzino L, Giammanco S (2019) Systematic review of geochemical data from thermal springs, gas vents and fumaroles of Southern Italy for geothermal favourability mapping. Earth-Science Rev 188:514–535. https://doi.org/10.1016/j.earscirev.2018.09.008
Mnjokava TT (2007) Interpretation of exploration geochemical data for geothermal fluids from the geothermal field of the rungwe Volcanic Area, SW Tanzania, UNU-GTP Reykjavík, Iceland.
Mtelela C (2018) Preliminary sedimentology and stratigraphy of the enigmatic middle lake beds succession (Pleistocene?) in the Rukwa Rift Basin. Tanzania Tanzania J Sci 44:75–96
Mtelela C, Roberts EM, Downie R, Hendrix MS (2016) Interplay of structural, climatic, and volcanic controls on late quaternary lacustrine-deltaic sedimentation patterns in the Western Branch of the East African Rift System, Rukwa Rift Basin. Tanzania J Sediment Res 86:1179–1207. https://doi.org/10.2110/jsr.2016.73
Nicholson K (1993) Geothermal fluids: Chemistry and exploration techniques. Springer-Verlag, Berlin Heidelberg. https://doi.org/10.1016/0375-6742(95)90013-6
Nzaro (1970) Geothermics (i97o)-SPECXAL xssu~ 2 U. N. Symposium on the Development and Utilization of Geothermal Resources, Pisa I97o 1039–1043.
Parkhurst DL, Appelo CAJ (2013) Description of Input and Examples for PHREEQC Version 3 — A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. U.S. Geological Survey Techniques and Methods, book 6, chapter A43, 497 p. U.S. Geol. Surv. Tech. Methods, B. 6, chapter A43 6–43A
Pisarskii BI, Konev AA, Levi KG, Delvaux D (1998) Alkaline carbon dioxide hydrotherms and strontium-containing travertines in the Songwe River valley (Tanzania). Geol. i Geofiz. 934–941.
Reed M, Nicolas S (1984) Calculation of pH and mineral equilibria in hydrothermal waters with application to geothermometry and studies of boiling and dilution. Geochim Cosmochim Acta 48:1479–1492
Roberts EM, O’Connor PM, Gottfried MD, Stevens N, Kapalima S, Ngasala S (2004) Revised stratigraphy and age of the Red Sandstone Group in the Rukwa Rift Basin. Tanzania Cretac Res 25:749–759. https://doi.org/10.1016/j.cretres.2004.06.007
Roberts EM, O’Connor PM, Stevens NJ, Gottfried MD, Jinnah ZA, Ngasala S, Choh AM, Armstrong RA (2010) Sedimentology and depositional environments of the Red Sandstone Group, Rukwa Rift Basin, southwestern Tanzania: new insight into cretaceous and Paleogene terrestrial ecosystems and tectonics in sub-equatorial Africa. J African Earth Sci 57:179–212. https://doi.org/10.1016/j.jafrearsci.2009.09.002
Semkiwa P, Kalkreuth W, Utting J, Mayagilo F, Mpanju F, Hagemann H (1998) The geology, petrology, palynology and geochemistry of Permian coal basins in Tanzania. 1. Namwele-Mkomolo, Muze and Galula coalfields. Int J Coal Geol 36:63–110. https://doi.org/10.1016/S0166-5162(97)00020-7
SWECO (1978) Reconnaissance of geothermal resources, Report for the Ministry of Water, Energy and Minerals of Tanzania, SWECO, Stockhlom, Sweden and VIRKIR, Reykjavik, Iceland.
Theunissen K, Klerkx J, Melnikov A, Mruma A (1996) Mechanisms of inheritance of rift faulting in the western branch of the East African Rift, Tanzania. Tectonics 15:776–790. https://doi.org/10.1029/95TC03685
Truesdell A, Jones B (1974) WATEQ: A computer program for calculating chemical equilibrium of natural waters. U.S.G.S. J Res 2:233–248
Wilson S (1961) pH of natural hydrothermal solution. Geochim Cosmochim Acta 25:233–235
Wopfner H, Kaaya CZ (1991) Stratigraphy and morphotectonics of Karoo deposits of the northern Selous Basin. Tanzania Geol Mag 128:319–334. https://doi.org/10.1017/S0016756800017593
Acknowledgements
The study is part of the NICHE Tanzania-Netherlands Energy Project. The Authors wish to thank the Tanzania Geothermal Development Company (TGDC) for providing previous geochemical data. We acknowledge all on-site support in Dar es Salaam and Mbeya region, which resulted in a successful field campaign.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich.
Author information
Authors and Affiliations
Contributions
S.N.A. performed data interpretation, modelling, and drafted the manuscript with input from all of the authors. M.N., M.B. and E.M. conducted field work. S.J. and A.M. provided previous geochemical data; M.B and G.B. acquired the funding and supervised the research. All together approved the final form of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asnin, S.N., Nnko, M., Josephat, S. et al. Identification of water–rock interaction of surface thermal water in Songwe medium temperature geothermal area, Tanzania. Environ Earth Sci 81, 513 (2022). https://doi.org/10.1007/s12665-022-10594-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-022-10594-4