Abstract
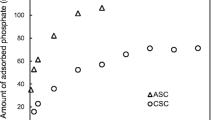
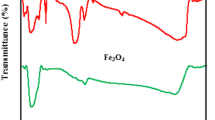
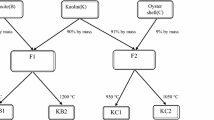
This study describes the development of scallop shell synthesized ceramic biomaterial for phosphorus removal from water. The synthesized biomaterial was characterized by scanning electron microscope, Brunauer–Emmett–Teller and X-ray diffractometer methods. The influences of contact time, initial phosphate concentration, initial solution pH, co-existing ions and temperature for phosphorus removal were investigated by batch experiments. The results indicated that the equilibrium data can be fitted by the Langmuir isotherm model at temperatures ranging from 15 to 55 °C, with the maximum sorption capacity of 13.6 mg/g. Sorption kinetics followed a pseudo-second-order kinetic equation model. The sorption process was optimal at a wide range of solution pH (above 2.4), with a relatively high sorption capacity level. Phosphorus sorption was slightly impeded by the presence of F−, HCO3 − and NH4 + ions, and significantly inhibited by Cl−, SO4 2− and NO3 − ions. Sorption process appeared to be controlled by a chemical precipitation processes. The mechanism may be attributed to ion complexation during subsequent sorption of phosphorus on scallop shell synthesized ceramic biomaterial.







Similar content being viewed by others
References
Akhurst DJ, Jones GB, Clark M, McConchie D (2006) Phosphate removal from aqueous solutions using neutralized bauxite refinery residues (Bauxsol(™)). Environ Chem 3:65–74
Blaney LM, Cinar S, Sengupta AK (2007) Hybrid anion exchanger for trace phosphate removal from water and wastewater. Water Res 41:1603–1613
Chen N, Zhang ZY, Feng CP, Sugiura N, Li M, Chen RZ (2010) Fluoride removal from water by granular ceramic adsorption. J Colloid Interface Sci 348:579–584
Chen N, Zhang ZY, Feng CP, Zhu DR, Yang YN, Sugiura N (2011) Preparation and characterization of porous granular ceramic containing dispersed aluminum and iron oxides as adsorbents for fluoride removal from aqueous solution. J Hazard Mater 186:863–868
Chubar NI, Kanibolotskyy VA, Strelko VV, Gallios GG, Samanidou VF, Shaposhnikova TO, Milgrandt VG, Zhuravlev IZ (2005) Adsorption of phosphate ions on novel inorganic ion exchangers. Colloids Surf 255:55–63
Das J, Patra BS, Baliarsingh N, Parida KM (2006) Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl Clay Sci 32:252–260
De-Bashana LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer. Water Res 38:4222–4246
Ennil-Köse T, Kιvanç B (2011) Adsorption of phosphate from aqueous solutions using calcined waste eggshell. Chem Eng J 178:34–39
Feng CP, Sugiura N, Shimada S, Maekawa T (2003) Development of a high performance electrochemical wastewater treatment system. J Hazard Mater 103(1–2):65–78
Freundlich HMF (1906) Uber die adsorption in losungen. Zeitschrift fur Physikalische Chemie 57:385–470
Fytianos K, Voudrias E, Raikos N (1998) Modeling of phosphorus removal from aqueous and wastewater samples using ferric iron. Environ Pollut 101:123–130
Georgantas DA, Grigoropoulou HP (2007) Orthophosphate and metaphosphate ion removal from aqueous solution using alum and aluminum hydroxide. J Colloid Interface Sci 315:70–79
Guo HM, Li Y, Zhao K, Ren Y, Wei C (2011) Removal of arsenite from water by synthetic siderite: behaviors and mechanisms. J Hazard Mater 186:1847–1854
Haghseresht F, Wang SB, Do DD (2009) A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl Clay Sci 46:369–375
Hanif A, Bhatti HN, Hanif MA (2009) Removal and recovery of Cu(II) and Zn(II) using immobilized Mentha arvensis distillation waste biomass. Ecol Eng 35:1427–1434
Ho YS, Mckay G (1999) Pseudo-second-order model for sorption process. Process Biochem 34:451–465
Huang W, Wang S, Zhu Z, Li L, Yao X, Rudolph V, Haghseresht F (2008) Phosphate removal from wastewater using red mud. J Hazard Mater 158:35–42
Huang X, Liao X, Shi B (2009) Adsorption removal of phosphate in industrial wastewater by using metal-loaded skin split waste. J Hazard Mater 166:1261–1265
Jeon DJ, Yeom SH (2009) Recycling wasted biomaterial, crab shells as an adsorbent for the removal of high concentration of phosphate. Bioresour Technol 100:2646–2649
Karaca S, Gürses A, Ejder M, Açιkyιldιz M (2006) Adsorptive removal phosphate from aqueous solutions using raw and calcined dolomite. J Hazard Mater B 128:273–279
Krishnan KA, Haridas A (2008) Removal of phosphate from aqueous solutions and sewage using natural and surface modified coir pith. J Hazard Mater 152:527–535
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part 1. Solids J Am Chem Soc 38:2221–2295
Leofanti G, Padovan M, Tozzola G, Venturelli B (1998) Surface area and pore texture of catalysts. Catal Today 41:207–219
Li Y, Liu C, Luan Z, Peng X, Zu C, Chen Z, Zhang Z, Fan J, Jia Z (2006) Phosphate removal from aqueous solutions raw and activated red mud and fly ash. J Hazard Mater 137:374–383
Li KQ, Li Y, Zheng Z (2010) Kinetics and mechanism studies of p-nitroaniline adsorption on activated carbon fibers prepared from cotton stalk by NH4H2PO4 activation and subsequent gasification with steam. J Hazard Mater 178:553–559
Liao XP, Ding Y, Wang B, Shi B (2006) Adsorption behavior of phosphate on metal-ions-loaded collagen fiber. Ind Eng Chem Res 45:3896–3901
Liu RX, Guo JL, Tang HX (2002) Adsorption of fluoride, phosphate, and arsenate ions an a new type of ion exchange fiber. J Colloid Interface Sci 248:268–274
Liu HL, Sun XF, Yin CG, Hu C (2008) Removal of phosphate by mesoporous ZrO2. J Hazard Mater 151:616–622
Lu SG, Bai SQ, Shan HD (2009) Removal mechanism of phosphate from aqueous solution by fly ash. J Hazard Mater 161:95–101
Morse GK, Brett SW, Guy JA, Lester JN (1998) Review: phosphorus removal and recovery technologies. Sci Total Environ 212:69–81
Mustafa S, Zaman MI, Khan S (2008) Temperature effect on the mechanism of phosphate anions sorption by β-MnO2. Chem Eng J 141:51–57
Namasivayam C, Sangeetha D (2004) Equilibrium and kinetics studies of adsorption of phosphate onto ZnCl2 activated coir pith carbon. J Colloid Interface Sci 280:359–365
National Environmental Protection Agency of China (1996) Integrated wastewater discharge standard (GB8978-1996), vol. 1. China Environmental Science Press, China, pp 1–20
Nowack B, Stone AT (2006) Competitive adsorption of phosphate and phosphonates onto goethite. Water Res 40:2201–2209
Razmovski R, Ściban M (2008) Biosorption of Cr(VI) and Cu(II) by waste tea fungal biomass. Ecol Eng 34:179–186
Rodrigues LA, Silva MCP (2009) Adsorção de ions fosfato em óxido de nióbio hidratado. Quim Nova 32:1206–1211
Shin EW, Han JS (2004) Phosphate adsorption on aluminum-impregnated mesoporous silicates: surface structure and behavior of adsorbents. Environ Sci Technol 38:912–917
Song D, Liu J, Huang F, Li GL, Meng L, Chen YH, Yu HR, Gao S, Gao WY (2013) Observation and characterization of micromorphology and microstructure of pyrite synthesized by hydrothermal method. Earth Sci Front 20(3):118–122
State Environmental Protection Administration (2002) Water and wastewater monitoring analysis method, 4th edn. China Environmental Science Press, China, vol. 4, pp 46–248
Swain SK, Tanushree P, Singh VK, Usha J, Patel RK, Dey RK (2011) Kinetics, equilibrium and thermodynamic aspects of removal of fluoride from drinking water using meso-structured zirconium phosphate. Chem Eng J 171:1218–1226
Tian SL, Jiang PX, Ning P, Su YH (2009) Enhanced adsorption removal of phosphate from water by mixed lanthanum/aluminum pillared montmorillonite. Chem Eng J 151:141–148
Tsai WT, Hsien KJ, Hsu HC, Lin CM, Lin KY, Chiu CH (2008) Utilization of ground eggshell waste as an adsorbent for the removal of dyes from aqueous solution. Bioresour Technol 99:1623–1629
Wang XB, Zhang SG, Gu MX, Li YL (2012) Discussions on the property of deep-lying groundwater resources in Hebei Plain. Earth Sci Front 19(6):243–247
Wang HR, Yang XX, Ding HR, Li Y, Zhu Y, Wang CQ, Lu AH (2013) The reduction effect of Cronobacter sakazakii on crystal structure of goethite. Earth Sci Front 20(3):147–153
Xiong J, He Z, Mahmood Q, Liu D, Yang X, Islam E (2008) Phosphate removal from solution using steel slag through magnetic separation. J Hazard Mater 152:211–215
Yildiz E (2004) Phosphate removal from water by fly ash using crossflow microfiltration. Sep Purif Technol 35:241–252
Zeng L, Li X, Liu J (2004) Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res 38:1318–1326
Zhang JD, Shen ZM, Shan WP, Wang WH (2011) Adsorption behavior of phosphate on lanthanum(III)-coordinated diamino-functionalized 3D hybrid mesoporous silicates material. J Hazard Mater 186:76–83
Zhou JB, Yang SL, Yu JG, Shu Z (2011) Novel hollow microspheres of hierarchical zinc–aluminum layered double hydroxides and their enhanced adsorption capacity for phosphate in water. J Hazard Mater 192:1114–1121
Acknowledgments
The authors thank “the Fundamental Research Funds for the Central Universities” (No. 2652013025), and the “National Natural Science Foundation” (No. 31140082) for financial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, N., Hu, W., Feng, C. et al. Removal of phosphorus from water using scallop shell synthesized ceramic biomaterials. Environ Earth Sci 71, 2133–2142 (2014). https://doi.org/10.1007/s12665-013-2618-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2618-2