Abstract
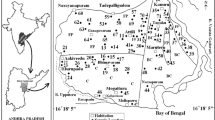
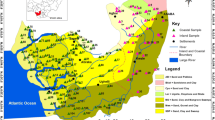
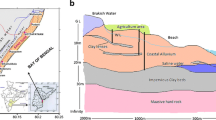
The assessment of hydrogeochemical processes that govern the water quality of inland freshwater aquifers in coastal environment, especially in Indian sub-continent, is occasionally attempted. To bridge the gap, a detail hydrochemical evaluation of groundwater occurring in coastal alluvium is attempted. Single set of high-density water sampling is done from a limited area to gain an in-depth knowledge of the processes that govern the water chemistry of the sandy aquifers. The water is of weak alkaline nature and less mineralized, EC being < 1,000 μS/cm in many samples. Major ion composition indicates that water is contaminated with excess concentration of nitrates. Ionic abundance is in the order of Cl− > Na+ > Ca2+ > HCO3 − > SO4 2− > Mg2+ > NO3 −. Na+ and Cl− are almost in similar proportions implying the influence of coastal climate on water quality. The water shows modest variation in their ionic assemblage among different sample points as evident from Schoeller scheme. Groundwater can be classified into three distinct facies viz. Cl−–Ca2+–Mg2+, Na+–Cl− and Ca2+–Mg2+–HCO3 − types. The ionic assemblages, their indices, ratios and cross-plots substantiate that multiple processes were involved in the evolution of the water chemistry. Among them, silicate weathering, halite dissolution, ion exchange and base exchange played prominent role in the ion enrichment of groundwater. The aquatic chemistry is further influenced and modified by marine environment, evapotranspiration and anthropogenic inputs which is authenticated by good correlation (r 2 = 1) among the Na+–Cl−, EC–Mg2+, Na+ and Cl−. Gibbs plots established that evaporation is more responsible for contribution of minerals to the groundwater than aquifer material. Nitrate contamination can be attributed for poor sewerage disposal mechanism which is aggravated by fertilizer inputs, irrigation practices and agriculture activity. A contrasting correlation (r 2 ≥90 to <0.40) among select pairs of ions reassures dissimilar source of those ions, involvement of multiple processes and limited interaction of formation water with aquifer material.















Similar content being viewed by others
References
Al-Agha MR, El-Nakhal HA (2004) Hydrochemical facies of groundwater in the Gaza Strip, Palestine. Hydrol Sci J des Sci Hydrol 49(3)
APHA (1992) Standard methods for the examination of water and wastewater, 17th edn. American Public Health Association, Washington, DC
Appleyard S (1995) The impact of urban development on recharge and groundwater quality in a coastal aquifer near Perth, Western Australia. Hydrogeol J 3(2):65–75. doi:10.1007/s100400050072
Ayers RS, Westcot DW (1985) Water quality for agriculture. FAO Irrigation and Drainage Paper No. 29, Rev.1
Barker PA, Newton JR, Bottrell HS, Tellam JH (1998) Processes affecting groundwater chemistry in a zone of saline intrusion into an urban sandstone aquifer. Appl Geochem 13(6):735–749. doi:10.1016/S0883-2927(98)00006-7
BIS (2003) IS 10500–91 (Revised 2003). Bureau of Indian standards for drinking water, New Delhi, pp 1–5
Böhlke JK, Denver JM (1995) Combined use of groundwater dating, chemical, and isotopic analyses to resolve the history and fate of nitrate contamination in two agricultural watersheds, Atlantic Coastal Plain, Maryland. Water Resour Res 31(9):2319–2339. doi:10.1029/95WR01584
Cerling TE, Pederson BL, Damn KLV (1989) Sodium–calcium ion exchange in the weathering of shales implication for global weathering budgets. Geology 17:552–554
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
Datta PS, Bhattacharya SK, Tyagi SK (1999) 18O studies on recharge of phreatic aquifers and groundwater flow-paths of mixing in the Delhi area. J Hydrol 176(1–4):25–36
Dutta PS, Deb DL, Tyagi SK (1997) Assessment of ground water contamination from fertilizers in Delhi area based on 18O, NO3 and K composition. J Contam Hydrol 27:249–262
Doneen LD (1964) Notes on water quality in Agriculture. Published as a water science and engineering paper 4001, Dept. of Water Science and Engineering, University of California
Eaton FM (1950) Significance of carbonate in irrigation water. Soil Sci 69(2):123–133
Gibbs RJ (1970) Mechanisms controlling world’s water chemistry. Science 170:1088–1090
HBoS-P (2007) Hand Book of Statistics: Prakasam District compiled and published by Chief Planning Officer, Prakasam District, Ongole. Govt., of Andhra Pradesh, India
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water: US Geol Surv Water Supply Paper-2254: 264, 3rd edn. Scientific Publishers, Jodhpur
Huh Y, Tsoi MY, Zaitiser A, Edward JN (1998) The fluvial geochemistry of the river of Eastern Siberia. 1. Tributaries of Lena river drainage the sedimentation platform of the Siberia Craton. Geochem Cosmochi Acts V 62:1657–1676
Jankowski J, Acworth RI (1997) Impact of debris-flow deposit on hydrogeochemical processes and the development of dry land salinity in the Yass River catchment, New South Wales, Australia. Hydrogeol J 5(44):71–88
Kalimas A, Gregorauskas M (2002) Ground water abstraction and contamination in Lithuania as geoindicators of environmental change. Environ Geol 42(7):767–772
Kass A, Gavrieli I, Yechieli Y, Vengosh A, Starinsky A (2005) The impact of freshwater and wastewater irrigation on the chemistry of shallow groundwater: a case study from the Israeli Coastal Aquifer. J Hydrol 300(1–4):314–331. doi:10.1016/j.jhydrol.2004.06.013
Kelley WP (1951) Alkali soils: their formation properties and reclamation. Reinold Publ Corp, New York
Kumar M, Ramanathan AL, Rao MS, Kumar Bhishm (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Enirn Geol 50:1025–1039
Kumar KP, Kumar M, Ramanathan AL, Tsujimur M (2009) Tracing the factors responsible for arsenic enrichment in groundwater of the middle Gangetic Plain, India: a source identification perspective. Environ Geochem Health. doi:10.1007/s10653-009-9270-5
Laluraj CM, Gopinath G (2006) Assessment on seasonal variation of groundwater quality of phreatic aquifers: a river basin system. Env Mont Asst 117(1–3):45–57. doi:10.1007/s10661-006-7675-5
Laluraj CM, Gopinath G, Kumar PKD (2005) Groundwater chemistry of shallow aquifers in the coastal zones of Cochin, India. Appl Ecol Environ Res (3): 133–139 http://drs.nio.org/drs/handle/2264/897
Liu W, Wang Z (2008) Groundwater chemistry in the Badain Jaran Desert Region of China. In: Bioinformatics and biomedical engineering, ICBBE 2008. The 2nd international conference. doi:10.1109/ICBBE.2008.589 (ISBN:978-1-4244-1747-6)
Lloyd JW, Heathcote JA (1985) Natural inorganic hydrochemistry in relation to ground water: an introduction. Oxford University Press, New York, p 296
Martínez D, Bocanegra E (2002) Hydrogeochemistry and cation-exchange processes in the coastal aquifer of Mar Del Plata, Argentina. Hydrogeol J 10(3):393–408. doi:10.1007/s10040-002-0195-7
May AL, Loucks MD (1995) Solute and isotope geochemistry and groundwater flow in the Central Wasatch Range, Utah. J Hydrol 170:795–840
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved leads. Am J Sci 287:401–428
Mills B (2003) Interpreting water for crop and posture: file no. F S 0334. DPI’S Agency for Food and Fiber Science, Toawoomba
Mitra BK, Sasaki C, Enari K, Matsuyama N, Fujita M (2007) Suitability assessment of shallow groundwater for agriculture in sand dune area of northwest Honshu island. Appl Ecol Environ Res 5(1):177–188. http://www.ecology.uni-corvinus.hu.ISSN 1589 1623 2007, Penkala Bt., Budapest, Hungary
Native R, Smith A (1987) Hydrgeology and geochemistry of the Ogallala aquifer southern high plians. J Hydrol 91:217–253
Park S-C, Yun S-T, Chae G-T, Yoo I-Y, Shin K-S, Heo C-H, Lee S-H (2005) Regional hydrochemical study on salinization of coastal aquifers, western coastal area of South Korea. J Hydrol 313(3–4):182–194. doi:10.1016/j.jhydrol.2005.03.001
Postma Dieke, Boesen Carsten, Kristiansen Henning, Larsen Flemming (1991) Nitrate reduction in an unconfined sandy aquifer: water chemistry, reduction processes, and geochemical modeling. Water Resour Res 27(8):2027–2045. doi:10.1029/91WR00989
Raju NJ (2011) Evaluation of hydrogeochemical processes in the Pleistacene aquifers of Middle Ganga Plain, Uttar Pradesh, India. Environ Earth Sci. doi:10.1007/s12665-011-1377-1
Raju NJ, Shukla UK, Ram P (2011) Hydrogeochemistry for the assessment of groundwater quality in Varanasi: a fast-urbanizing center in Uttar Pradesh, India. Environ Monit Assess. doi:10.1007/s10661-010-1387-6
Rao CHH (1994) Agricultural development and environmental degradation: an analytical frame work at Pg No. 160 in book In: Agricultural growth rural poverty and environmental degradation in India© Institute of Economic growth. ISBN 0 19 563343 1
Rao NS (2007) Groundwater quality as a factor for identification of recharge zones. Environ Geosci 14:79–90
Rao NS (2008) Factors controlling the salinity in groundwaters from a part of Guntur district, Andhra Pradesh, India. Environ Monit Assess 138:327–341
Rao NS, Rao PS (2009) Major ion chemistry of groundwater in a river basin: a study from India. Environ Earth Sci. doi:10.1007/s12665-009-0389-6
Rashid U, Izrar A (2007) Hydrochemical characteristics of groundwater in parts of Kushva–Yamuna basin, Muzaffarnagar district, UP. J Geol Soc India 69:970–982
Reddy AGS (2009) Ground water Management studies of N and NE parts of Prakasam district, AP. Report of Central Ground Water Board, Southern Region
Reddy DV, Nagabhushanam P, Sukhija PS, Reddy AGS, Smedley PL (2010) Fluoride dynamics in the granitic aquifer of the Wailapally watershed, Nalgonda District, India. Chem Geol 269:278–289. doi:10.1016/j.chemgeo.2009.10.003
Ronen D, Kanfi Y, Magaritz M (1983) Sources of nitrates in groundwater of the coastal plain of Israel evolution of ideas. Water Research 17(11):1499–1503. doi:10.1016/0043-1354(83)90004-0
Rupert, MG, Plummer LN (2004) Ground-water flow direction, water quality, recharge sources, and age, great sand dunes national monument, South-Central Colorado, 2000–2001. In: U.S. Geological Survey Scientific Investigations Report 2004–5027, p 28
Sami K (1992) Recharge mechanism and geochemical processes in a semi arid sedimentary basin, Eastern Cape, South Africa. J Hydrol 139:27–48
Sarin MM, Krishnaswmay S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga–Brahmaputra river system: weathering processes and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009
Schoeller H (1965) Hydrodynamique lans lekarst (ecoulemented emmagusinement). Actes Colloques Doubronik, I: AIHS et UNESCO, pp 3–20
Schoeller H (1967) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigation and development. Water research, Series-33: UNESCO, pp 44–52
Singh P, Kunwar AM, Mohan D, Singh VK, singh S (2006) Evaluation of groundwater quality in northern Indo-Gangetic alluvium region. Envirn Monit Assess 112:211–230. doi:10.1007/s10661-006-0357-5
Soltan ME (1998) Characterization, classification and evaluation of some groundwater samples in Upper Epygt. Chemos 37:735–747
Soltan ME (1999) Evaluation of groundwater quality in Dakhla Oasis (Egyptian Western Desert). Eviron Monit Assess 57:157–168
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon, the influence of geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Stuyfzand JP (1999) Patterns in groundwater chemistry resulting from groundwater flow. Hydrogeol J 7(1):15–27. doi:10.1007/s100400050177
Subramanian V, Saxena K (1983) Hydrogeochemistry of groundwater in the Delhi region of India, relation of water quality and quantity. In: Proceedings of the Hamberg symposium IAHS publication no. 146, pp 307–316
Todd DK (1980) Groundwater hydrology. Wiley, Singapore
USGS (2000) http://pubs.usgs.gov/fs/2000/fs-057-00/pdf/fs05700.pdf. Accessed May 2012.
Wen X, Wu Y, Su J, Zhang Y, Liu F (2005) Hydrochemical characteristics and salinity of groundwater in the Ejina Basin, Northwestern China. Environ Geol :665–675. doi:10.1007/s00254-005-0001-7
Zhang J, Huang WW, Letolle R, Jusserand C (1995) Major element chemistry of the Huanghe (Yellow River), China-weathering processes and chemical fluxed. J Hydrol 168:173–203
Zuber A, Michalczyk Z, Maloszewski P (2001) Great tritium ages explain the occurrence of good-quality groundwater in a phreatic aquifer of an urban area, Lublin, Poland. Hydrogeol J 9(5):451–460. doi:10.1007/s100400100149
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reddy, A.G.S. Evaluation of hydrogeochemical characteristics of phreatic alluvial aquifers in southeastern coastal belt of Prakasam district, South India. Environ Earth Sci 68, 471–485 (2013). https://doi.org/10.1007/s12665-012-1752-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1752-6