Abstract
Background
Though a few studies in animal models suggest that intestinal helminths (IH) favorably affect evolution of gastritis associated with Helicobacter pylori (H. pylori) the studies supporting this concept in humans are only a few and are based on serological data.
Methods
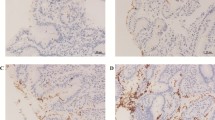
To evaluate the possible influence of IH on the human gastric mucosa, three groups of Venezuelan adults with gastropathy (endoscopically diagnosed) were studied: H. pylori−/IH− (n = 17), H. pylori+/IH− (n = 18), and H. pylori+/IH+ (n = 11). Histological analysis (hematoxylin-eosin) and immunohistochemical staining (peroxidase) for cytokines interleukin-1beta (IL-1β), tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and interleukin 4 (IL-4) were undertaken in gastric antral biopsies.
Results
Expression of the four cytokines was detected in all individuals in varying degrees, but proinflammatory cytokines were expressed in a higher degree in the H. pylori+/IH− group, mainly IL-1β (Th1-dominant immune response), associated with a higher degree of both histological inflammation and gastric cancer risk index (GCRI), as compared to the H. pylori−/IH− group. In contrast, an increased expression of IL-4 and a reduced expression of proinflammatory cytokines (Th2-dominant response), plus the tendency to a lower degree of mononuclear infiltration, mucosal atrophy in gastric corpus, and GCRI, were evidenced in the coinfected group.
Conclusions
The findings of the present study is perhaps the first histological evidence of a possible modulatory effect of IH on the gastric mucosal inflammatory response due to H. pylori infection in humans.






Similar content being viewed by others
References
Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119:2475–87.
Oluwasola AO. Genetic determinants and clinico-pathological outcomes of Helicobacter pylori infection. Ann Ib Postgrad Med. 2014;12:22–30.
Fox JG, Beck P, Dangler CA, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces Helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–42.
Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Microbiol Rev. 2010;23:713–39.
Rogers AB. Gastric Helicobacter spp. in animal models: pathogenesis and modulation by extragastric coinfections. Methods Mol Biol. 2012;921:175–88.
Whary MT, Muthupalani S, Ge Z, et al. Helminth co-infection in Helicobacter pylori infected INS-GAS mice attenuates gastric premalignant lesions of epithelial dysplasia and glandular atrophy and preserves colonization resistance of the stomach to lower bowel microbiota. Microbes Infect. 2014;16:345–55.
Martin HR, Shakya KP, Muthupalani S, et al. Brugia filariasis differentially modulates persistent Helicobacter pylori gastritis in the gerbil model. Microbes Infect. 2010;12:748–58.
Whary MT, Sundina N, Bravo LE, et al. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol Biomark Prev. 2005;14:1464–9.
Ek C, Whary MT, Ihrig M, Bravo LE, Correa P, Fox JG. Serologic evidence that Ascaris and Toxoplasma infections impact inflammatory responses to Helicobacter pylori in Colombians. Helicobacter. 2012;17:107–15.
Arismendi-Morillo G, Hernández I, Mengual E, Fuenmayor A, Romero G, Lizarzábal M. Comparison of three methods based on endoscopic gastric biopsies for diagnosis of Helicobacter pylori active infection in a clinical setting. Arq Gastroenterol. 2011;48:190–4.
Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The Updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81.
Meining A, Bayerdörffer E, Müller P, et al. Gastric carcinoma risk index in patients infected with Helicobacter pylori. Virchows Arch. 1998;432:311–4.
Serrano C, Diaz MI, Valdivia A, et al. Relationship between Helicobacter pylori virulence factors and regulatory cytokines as predictors of clinical outcome. Microbes Infect. 2007;9:428–34.
Dhakhwa R, Acharya IL, Shrestha HG, Joshi DM, Lama S, Lakhey M. Histopathologic study of chronic antral gastritis. J Nepal Health Res Counc. 2012;10:57–60.
Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Comparison of Helicobacter pylori infection and gastric mucosal histological features of gastric ulcer patients with chronic gastritis patients. World J Gastroenterol. 2005;11:976–81.
Lamarque D, Tran Van Nhieu J, Breban M. What are the gastric modifications induced by acute and chronic Helicobacter pylori infection? Gastroenterol Clin Biol. 2003;27:391–400.
Vega-Ramos B, Rodríguez-Moguel L. Helicobacter pylori. Its relationships and morphological controversies. Rev Gastroenterol Mex. 2000;65 4 Suppl 2:34–40.
Lopes AI, Quiding-Jarbrink M, Palha A, et al. Cytokine expression in pediatric Helicobacter pylori infection. Clin Diagn Lab Immunol. 2005;12:994–1002.
Strömberg E, Edebo A, Svennerholm AM, Lindholm C. Decreased epithelial cytokine responses in the duodenal mucosa of Helicobacter pylori-infected duodenal ulcer patients. Clin Diagn Lab Immunol. 2003;10:116–24.
Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjets. Infect Immun. 1998;66:5964–71.
D’Elios MM, Manghetti M, De Carli M, et al. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–7.
Bamford KB, Fan X, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–92.
D’Elios MM, Amedei A, Benagiano M, Azzurri A, Del Prete G. Helicobacter pylori, T cells and cytokines: the ‘dangerous liaisons’. FEMS Immunol Med Microbiol. 2005;44:113–9.
Amedei A, Cappon A, Codolo G, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092–101.
Pellicanò A, Imeneo M, Leone I, Larussa T, Luzza F. Enhanced activation of cyclooxygenase-2 downregulates Th1 signaling pathway in Helicobacter pylori-infected human gastric mucosa. Helicobacter. 2007;12:193–9.
Bimczok D, Clements RH, Waites KB, et al. Human primary gastric dendritic cells induce a Th1 response to H. pylori. Mucosal Immunol. 2010;3:260–9.
Uehara A, Okumura T, Sekiya C, Okamura K, Takasugi Y, Namiki M. Interleukin-1 inhibits the secretion of gastric acid in rats: possible involvement of prostaglandin. Biochem Biophys Res Commun. 1989;162:1578–84.
Datta De D, Bhattacharjya S, Maitra M, et al. IL1β induced Smad 7 negatively regulates gastrin expression. PLoS One. 2011;6:e14775.
Nutman TB. Looking beyond the induction of Th2 responses to explain immunomodulation by helminthes. Parasite Immunol. 2015;37:304–13.
Maizels RM, Smits HH, McSorley HJ. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity. 2018;49:801–18.
Eichenberger RM, Ryan S, Jones L, et al. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front Immunol. 2018;9:850.
Whary MT, Fox JG. Th1-mediated pathology in mouse models of human disease is ameliorated by concurrent Th2 responses to parasite antigens. Curr Top Med Chem. 2004;4:531–8.
Moreau E, Chauvin A. Immunity against helminths: interactions with the host and the intercurrent infections. J Biomed Biotechnol. 2010;428593.
Acknowledgments
The authors thank doctors Jesús Mosquera, Hector Pons, and Yasmir Quiroz for the counseling for quantitation of cytokines. The authors also thank Gladys Colina, Nivia Abreu, and Neyla Molero de Bohórquez for the technical collaboration.
Funding
This work was partially supported by the Scientific and Humanistic Development Council of the Universidad del Zulia, Venezuela (Subvention No. CC-0660-10).
Author information
Authors and Affiliations
Contributions
Alisbeth Fuenmayor Boscán: study concept and protocol design; collecting data; analysis of data; preparing the initial draft of the manuscript; obtained funding.
Ileana Hernández-Rincón: study concept and protocol design; critical revision of the manuscript for intellectual content; study supervision.
Gabriel Arismendi-Morillo, Edgardo Mengual, Zulbey Rivero, Gisela Romero, and Maribel Lizarzábal: technical, administrative and material support.
Melchor Álvarez-Mon: study concept and protocol design; critical revision of the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
AF-B, IH-R, GA-M, EM, ZR, GR, ML, and MA-M declare that they have no conflict of interest.
Ethics statement
The study was performed conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fuenmayor-Boscán, A., Hernández-Rincón, I., Arismendi-Morillo, G. et al. Changes in the severity of gastric mucosal inflammation associated with Helicobacter pylori in humans coinfected with intestinal helminths. Indian J Gastroenterol 39, 186–195 (2020). https://doi.org/10.1007/s12664-020-01023-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-020-01023-0