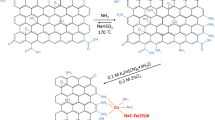
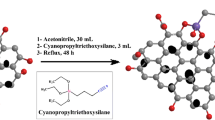
Abstract
A new class of polyethylene glycol/graphene/carbon nanotube (PEG/G/CNTa–e) composites were prepared using a simple dissolution process, with ultrasonic assistance. Mixed G/CNT, with variable loadings, have been utilized as reinforcements for a PEG polymer matrix, to produce a new class of nanocomposite materials. The PEG/G/CNT composite was characterized using various spectroscopic techniques, such as Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), and transmission electron microscopy (TEM), to identify various bonds and to confirm the structure of the materials. To understand the potentiality of the material, the selectivity of PEG/G/CNT towards different metal ions, including Au(III), Y(III), Pb(II), Zn(II), Cu(II), Ni(II), Fe(III), Cr(III), and Co(II), was evaluated. The selectivity of the PEG/G/CNT e-phase was strongest towards Au(III). PEG/G/CNT was utilized as a particle extractor for Au(III) discovery in watery media. The static adsorption limit of PEG/G/CNT for Au(III) was determined to be 80.80 mg/g. The results of the adsorption isothermal assay additionally affirmed the legitimacy of the Langmuir isotherm model for the adsorption procedure. In addition, the productivity of recently incorporated materials was examined for the specific extraction of Au(III), using inductively coupled plasma-optical emission spectrometry (ICP-OES). This PEG/G/CNTa–e material can be used for the extraction of gold from the abundantly minerals.
Graphic Abstract








Similar content being viewed by others
References
Niu, X., et al.: Electrochemical DNA sensor based on chitosan-graphene and electrodeposited gold nanoparticle modified electrode for the detection of Staphylococcus aureus nuc gene sequence. Curr. Anal. Chem. 14, 159–165 (2018)
Koshy, O., Pottathara, Y.B., Thomas, S., Petovar, B., Finsgar, M.A.: Flexible, disposable hydrogen peroxide sensor on graphene nanoplatelet-coated cellulose. Curr. Anal. Chem. 13, 480 (2017)
Niu, X., et al.: Direct electrochemistry of myoglobin on three-dimensional graphene- nickel oxide modified electrode and electrocatalytic detection of trichloroacetic acid. Curr. Anal. Chem. 13, 410 (2017)
Sayar, O., et al.: Optimization of solid-phase extraction by experimental design methodology for determination of lead ions using graphene modified nano-sheets as a novel sorbent. Curr. Anal. Chem. 10, 512–521 (2014)
Fan, Z.-J., Yan, J., Wei, T., Ning, G.-Q., Zhi, L.-J., Liu, J.-C., Cao, D.-X., Wang, G.-L.: Nanographene-constructed carbon nanofibers grown on graphene sheets by chemical vapor deposition: high-performance anode materials for lithium ion batteries. ACS Nano 5, 2787–2794 (2011)
Yan, J., et al.: Electrochemical properties of graphene nanosheet/carbon black composites as electrodes for supercapacitors. Carbon N. Y. 48, 1731–1737 (2010)
Yoo, E., et al.: Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 8, 2277–2282 (2008)
Yu, K., Lu, G., Bo, Z., Mao, S., Chen, J.: Carbon nanotube with chemically bonded graphene leaves for electronic and optoelectronic applications. J. Phys. Chem. Lett. 2, 1556–1562 (2011)
Lee, J., Park, E.J., Choi, J., Hong, J., Shim, S.E.: Polyurethane/PEG-modified MWCNT composite film for the chemical vapor sensor application. Synth. Met. 160, 566–574 (2010)
Mohammadi, S.Z., Afzali, D., Pourtalebi, D.: Flame atomic absorption spectrometric determination of trace amounts of lead, cadmium and nickel in different matrixes after solid phase extraction on modified multiwalled carbon nanotubes. Cent. Eur. J. Chem. 8, 662–668 (2010)
Li, Y., Hu, B.: Cloud point extraction with/without chelating agent on-line coupled with inductively coupled plasma optical emission spectrometry for the determination of trace rare earth elements in biological samples. J. Hazard. Mater. 174, 534–540 (2010)
Bonfil, Y., Kirowa-Eisner, E.: Determination of nanomolar concentrations of lead and cadmium by anodic-stripping voltammetry at the silver electrode. Anal. Chim. Acta 457, 285–296 (2002)
Tanikkul, S., et al.: Flow injection in-valve-mini-column pretreatment combined with ion chromatography for cadmium, lead and zinc determination. Talanta 64, 1241–1246 (2004)
Ai, S., Xu, Q.: Separation of inorganic anions and cations by high-speed ion chromatography. Curr. Anal. Chem. 2, 389–396 (2006)
Wu, A., et al.: Review the application of chromatography in the analysis of nitric oxide-derived nitrite and nitrate ions in biological fluids. Curr. Anal. Chem. 10, 609–621 (2014)
Pyrzyńska, K.: Recent developments in the determination of gold by atomic spectrometry techniques. Spectrochim. Acta B At. Spectrosc. 60, 1316–1322 (2005)
Nasu, A., Yamauchi, S., Sekine, T.: Solvent extraction of Copper(I) and (II) as thiocyanate complexes with tetrabutylammonium ions into chloroform and with trioctylphosphine oxide into hexane. Anal. Sci. 13, 903–911 (1997)
Tao, G., Fang, Z.: Dual stage preconcentration system for flame atomic absorption spectrometry using flow injection on-line ion-exchange followed by solvent extraction. Fresenius. J. Anal. Chem. 360, 156–160 (1998)
Soylak, M., Erdogan, N.D.: Copper(II)–rubeanic acid coprecipitation system for separation–preconcentration of trace metal ions in environmental samples for their flame atomic absorption spectrometric determinations. J. Hazard. Mater. 137, 1035–1041 (2006)
Saracoglu, S., Yilmaz, E., Soylak, M.: Speciation of chromium after coprecipitation with Cu-violuric acid and determination by flame atomic absorption spectrometry. Curr. Anal. Chem. 8, 358–364 (2012)
Manzoori, J.L., Abdolmohammad-Zadeh, H., Amjadi, M.: Simplified cloud point extraction for the preconcentration of ultra-trace amounts of gold prior to determination by electrothermal atomic absorption spectrometry. Microchim. Acta 159, 71–78 (2007)
Ahmed, S.A.: Alumina physically loaded by thiosemicarbazide for selective preconcentration of mercury(II) ion from natural water samples. J. Hazard. Mater. 156, 521–529 (2008)
Montero Alvarez, A., Estévez Alvarez, J.R., Padilla Alvarez, R.: Heavy metal analysis of rainwaters a comparison of TXRF and ASV analytical capabilities. J. Radioanal. Nucl. Chem. 273, 427–433 (2007)
Li, P., Pei, F., Liu, Q., Fang, Y.: Magnetic solid-phase extraction for the determination of ochratoxin A in wine and beer by HPLC-FLD. Curr. Anal. Chem. 14, 129–134 (2018)
Al-Angari, Y., Kadi, M., Ismail, I., Gabal, M.: Effect of alumina incorporation on restricting grain growth of nanocrystalline tin(IV) oxide. Open Chem. 8, 331 (2010)
Pei, S., Fang, Z.: Flame atomic absorption spectrometric determination of silver in geological materials using a flow-injection system with on-line preconcentration by coprecipitation with diethyldithiocarbamate. Anal. Chim. Acta 294, 185–193 (1994)
Cho, H.-J., Myung, S.-W.: Determination of cadmium, chromium and lead in polymers by ICP-OES using a high pressure asher (HPA). Bull. Korean Chem. Soc. 32, 489–497 (2011)
de Castro, G.R., et al.: Synthesis, characterization and determination of the metal ions adsorption capacity of cellulose modified with p-aminobenzoic groups. Mater. Res. 7, 329–334 (2004)
Liu, Y., Guo, L., Zhu, L., Sun, X., Chen, J.: Removal of Cr(III, VI) by quaternary ammonium and quaternary phosphonium ionic liquids functionalized silica materials. Chem. Eng. J. 158, 108–114 (2010)
Awwad, N.S., Gad, H.M.H., Ahmad, M.I., Aly, H.F.: Sorption of lanthanum and erbium from aqueous solution by activated carbon prepared from rice husk. Colloids Surf. B Biointerfaces 81, 593–599 (2010)
Marwani, H.M., Albishri, H.M., Soliman, E.M., Jalal, T.A.: Selective adsorption and determination of hexavalent chromium in water samples by chemically modified activated carbon with tris(hydroxymethyl)aminomethane. J. Dispers. Sci. Technol. 33, 549–555 (2012)
Biparva, P., Hadjmohammadi, M.R.: Selective separation/preconcentration of silver ion in water by multiwalled carbon nanotubes microcolumn as a sorbent. Clean: Soil, Air, Water 39, 1081–1086 (2011)
Abd El-Hay, S.S., Aldawsari, H., Gouda, A.A.: a new separation and enrichment method of heavy metals in water and food samples using 2-(2’-benzothiazolylazo)-6-aminophenol impregnated multi-walled carbon nanotubes. Curr. Anal. Chem. 14, 120–128 (2018)
Jung, D.-H., Koan Ko, Y., Jung, H.-T.: Aggregation behavior of chemically attached poly(ethylene glycol) to single-walled carbon nanotubes (SWNTs) ropes. Mater. Sci. Eng. C 24, 117–121 (2004)
Liu, W., et al.: PEG-assisted synthesis of homogeneous carbon nanotubes-MoS2-carbon as a counter electrode for dye-sensitized solar cells. Electrochim. Acta 144, 119–126 (2014)
Hussein, A.: The role of mixed graphene/carbon nanotubes on the coating performance of G/CNTs/epoxy resin nanocomposites. Int. J. Electrochem. Sci. (2016). https://doi.org/10.20964/2016.09.13
Abdel Salam, M., Burk, R.: Novel application of modified multiwalled carbon nanotubes as a solid phase extraction adsorbent for the determination of polyhalogenated organic pollutants in aqueous solution. Anal. Bioanal. Chem. 390, 2159–2170 (2008)
Naebe, M., et al.: Mechanical property and structure of covalent functionalised graphene/epoxy nanocomposites. Sci. Rep. 4, 4375 (2015)
Lawal, A.T.: Synthesis and utilisation of graphene for fabrication of electrochemical sensors. Talanta 131, 424–443 (2015)
Feng, W., et al.: Well-aligned polyaniline/carbon-nanotube composite films grown by in-situ aniline polymerization. Carbon N. Y. 41, 1551–1557 (2003)
Rahman, M.M., Hussein, M.A., Alamry, K.A., Al-Shehry, F.M., Asiri, A.M.: Polyaniline/graphene/carbon nanotubes nanocomposites for sensing environmentally hazardous 4-aminophenol. Nano-Struct. Nano-Objects 15, 63–74 (2018)
Feng, L., et al.: The shape-stabilized phase change materials composed of polyethylene glycol and various mesoporous matrices (AC, SBA-15 and MCM-41). Sol. Energy Mater. Sol. Cells 95, 3550–3556 (2011)
Zhang, L., Zhu, J., Zhou, W., Wang, J., Wang, Y.: Thermal and electrical conductivity enhancement of graphite nanoplatelets on form-stable polyethylene glycol/polymethyl methacrylate composite phase change materials. Energy 39, 294–302 (2012)
Feng, L., et al.: Preparation and characterization of polyethylene glycol/active carbon composites as shape-stabilized phase change materials. Sol. Energy Mater. Sol. Cells 95, 644–650 (2011)
Zhang, L., Zhu, J., Zhou, W., Wang, J., Wang, Y.: Characterization of polymethyl methacrylate/polyethylene glycol/aluminum nitride composite as form-stable phase change material prepared by in situ polymerization method. Thermochim. Acta 524, 128–134 (2011)
Wang, C., et al.: Graphene oxide stabilized polyethylene glycol for heat storage. Phys. Chem. Chem. Phys. 14, 13233 (2012)
Liew, K.M., Lei, Z.X., Zhang, L.W.: Mechanical analysis of functionally graded carbon nanotube reinforced composites: a review. Compos. Struct. 120, 90–97 (2015)
Han, D.-M., Fang, G.-Z., Yan, X.-P.: Preparation and evaluation of a molecularly imprinted sol–gel material for on-line solid-phase extraction coupled with high performance liquid chromatography for the determination of trace pentachlorophenol in water samples. J. Chromatogr. A 1100, 131–136 (2005)
Senturk, H.B., et al.: Separation and enrichment of gold(III) from environmental samples prior to its flame atomic absorption spectrometric determination. J. Hazard. Mater. 149, 317–323 (2007)
Liang, P., Zhao, E., Ding, Q., Du, D.: Multiwalled carbon nanotubes microcolumn preconcentration and determination of gold in geological and water samples by flame atomic absorption spectrometry. Spectrochim. Acta B At. Spectrosc. 63, 714–717 (2008)
Zhang, L., Li, Z., Du, X., Chang, X.: Activated carbon functionalized with 1-amino-2-naphthol-4-sulfonate as a selective solid-phase sorbent for the extraction of gold(III). Microchim. Acta 174, 391–398 (2011)
Albishri, H.M., Marwani, H.M.: Chemically modified activated carbon with tris(hydroxymethyl)aminomethane for selective adsorption and determination of gold in water samples. Arab. J. Chem. 9, S252–S258 (2016)
Rahman, M.M., et al.: Selective determination of gold(III) ion using CuO microsheets as a solid phase adsorbent prior by ICP-OES measurement. Talanta 104, 75–82 (2013)
Marwani, H.M., Albishri, H.M., Jalal, T.A., Soliman, E.M.: Activated carbon immobilized dithizone phase for selective adsorption and determination of gold(III). Desalin. Water Treat. 45, 128–135 (2012)
Marwani, H.M., et al.: Selective detection of gold(III) ions based on codoped MnO2–SnO2 nanocubes prepared by solution method. Mater. Res. Bull. 51, 287–294 (2014)
Langmuir, I.: The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 38, 2221–2295 (1916)
McKay, G., Blair, H.S., Gardner, J.R.: Adsorption of dyes on chitin. I. Equilibrium studies. J. Appl. Polym. Sci. 27, 3043–3057 (1982)
Acknowledgements
This project was funded by Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under Grant No. (DF-235-130-1441). The authors, therefore, gratefully acknowledge DSR technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, A., Hussein, M.A., Alsheri, F.M. et al. A New Class of Polyethylene Glycol-Grafted Graphene Carbon Nanotube Composite as a Selective Adsorbent for Au(III). Waste Biomass Valor 12, 937–946 (2021). https://doi.org/10.1007/s12649-020-01053-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01053-x