Abstract
Purpose
The effect of volatile anesthetics on the mechanism(s) of vascular contraction in diabetes mellitus (DM) has not been fully understood. The current study was designed to determine the effects of sevoflurane on the norepinephrine (NE)-induced changes in contractile state and intracellular Ca2+ concentrations ([Ca2+]i) in the spontaneously developing type 2 DM rat.
Methods
The effects of sevoflurane on NE (10−6M)-induced vasoconstriction and increase in [Ca2+]i in the aortas from Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a type 2 DM model, and from age-matched control Long-Evans Tokushima Otsuka (LETO) rats were investigated using an isometric force transducer and fluorometer with fura-2 as an indicator of [Ca2+]i.
Results
Norepinephrine-induced increases in tension and [Ca2+]i in OLETF rats were 54.8%, 95% confidence interval (CI) 36.9-72.6% and 58.8%, 95% CI 51.5-66.1%, respectively, and in LETO rats they were 46.4%, 95% CI 39.0-53.7% and 53.8%, 95% CI 46.9-60.7%, respectively, when expressed as the percentage relative to that induced by KCl 30 mM. In LETO rats, sevoflurane at a concentration of 3.4% inhibited the vascular contraction (9.4%, 95% CI 6.3-12.6%; P < 0.001) and the increase in [Ca2+]i (33.3%, 95% CI 27.4-39.2%; P = 0.002). In OLETF rats, however, sevoflurane failed to affect either the NE-induced contraction (43.6%, 95% CI 28.3-58.9%; P = 0.68) or the elevation in [Ca2+]i (60.5%, 95% CI 56.3-64.8%; P = 0.93).
Conclusion
Sevoflurane at clinically relevant concentrations inhibited the NE-induced increase in [Ca2+]i in the aortic smooth muscle from normal rats but not in that from type 2 DM rats. Thus, a Ca2+- signalling pathway resistant to sevoflurane appears to exist in the type 2 DM rat aorta.
Resumé
Objectif
L’effet des anesthésiques volatils sur les mécanismes de contraction vasculaire en présence de diabète sucré n’est pas encore tout à fait compris. L’étude en cours a été conçue afin de déterminer les effets du sévoflurane sur les changements de contractilité et de concentration intracellulaire de Ca2+ ([Ca2+]i) induits par la norépinephrine (NE) en cas de développement spontané du diabète sucré de type 2 chez le rat.
Méthodes
Les effets du sévoflurane sur la vasoconstriction induite par la NE (10−6M) et sur l’augmentation de [Ca2+]i dans l’aorte de rats OLETF (Otsuka Long-Evans Tokushima Fatty) présentant un diabète sucré de type 2 ont été comparés aux effets observés chez des rats LETO (Long-Evans Tokushima Otsuka) témoins appariés en fonction de l’âge, à l’aide d’un transducteur de force isométrique et d’un fluoromètre utilisant du fura-2 comme mesure de [Ca2+]i.
Résultats
La NE a entraîné des augmentations de la tension de 54,8 % (intervalle de confiance [IC] à 95 %: 36,9 à 72,6 %) et de [Ca2+]i de 58,8 % (IC 95 %: 51,5 à 66,1 %) chez les rats OLETF, tandis que pour les rats LETO, ces augmentations étaient de 46,4 %, (IC 95 %: 39,0 à 53,7 %) et de 53,8 %, (IC 95 %: 46,9 à 60,7 %), respectivement, lorsque exprimées sous forme de pourcentage relatif aux augmentations induites par 30 mM de KCl. Chez les rats LETO, le sévoflurane à une concentration de 3,4 % a inhibé la contraction vasculaire (9,4 %; IC 95 %: 6,3 à 12,6 %; P < 0,001) et l’augmentation de [Ca2+]i (33,3 %, IC 95 %: 27,4 à 39,2 %; P = 0,002). Cependant, chez les rats OLETF, le sévoflurane n’a eu aucune incidence sur les changements induits par la NE, c’est-à-dire la contraction vasculaire (43,6 %; IC 95 %: 28,3 à 58,9 %; P = 0,68) ou l’augmentation de [Ca2+]i (60,5 %; IC 95 %: 56,3 à 64,8 %; P = 0,93).
Conclusion
À des concentrations pertinentes sur le plan clinique, le sévoflurane a inhibé l’augmentation induite par la NE de [Ca2+]i dans le muscle lisse vasculaire de l’aorte chez les rats normaux, mais pas chez les rats présentant un diabète sucré de type 2. Par conséquent, une voie de signalisation Ca2+- résistante au sévoflurane semble exister dans l’aorte des rats présentant un diabète sucré de type 2.
Similar content being viewed by others
Diabetes mellitus (DM) is a common disease that is rapidly increasing worldwide. Due to the increasing prevalence of diabetic patients undergoing surgery and the hemodynamic instability in diabetic patients during surgery,1 it is essential for anesthesiologists to understand the pathophysiology of cardiovascular changes and the altered response to anesthetics in DM. Cardiovascular complications are recognized to be the leading cause of morbidity and mortality in patients with DM,2 and the complications are thought to be caused, at least in part, by abnormal vascular reactivity.3
This possibility of abnormal vascular reactivity in DM has been investigated mainly in arterial preparations from streptozotocin (STZ)-treated rats, which are considered models of poorly-controlled type 1 DM. However, in 2008, the World Health Organization reported that more than 180 million people worldwide have DM and 90% of those have type 2 DM. Moreover, quantitative and qualitative differences in cardiovascular dysfunction have been reported between animal models of insulin-dependent DM and animal models of non-insulin-dependent DM.4
We have previously reported that anesthetic induction with sevoflurane increased the skin temperature in non-DM patients to a greater extent than in type 2 DM patients.5 Although these findings suggest altered vascular reactivity to volatile anesthetics in diabetic patients, the differences in vascular reactivity in response to anesthetics between DM and non-DM patients and the underlying mechanisms have not yet been determined.
The aim of the present study was to investigate and compare the effects of sevoflurane on norepinephrine (NE)-induced changes in the contractile state and the intracellular Ca2+ concentrations ([Ca2+]i) of aortic smooth muscle from a spontaneously developing type 2 DM rat model and from their genetic controls.
Methods
Animals and experimental design
For this study, we used Otsuka Long-Evans Fatty (OLETF) rats, which have been characterized as a genetic model of spontaneously developing type 2 DM and their genetic controls, Long-Evans Tokushima Otsuka (LETO) rats. Five-week-old male rats of both strains were supplied by the Tokushima Research Institute (Otsuka Pharmaceutical, Tokushima, Japan). The rats were housed in temperature-controlled cages with a 12-hr light-dark cycle, and they were given free access to food and water until they were 45-50 weeks old. The protocol was approved by the Wakayama Medical University Animal Care and Use Committee, and all studies were conducted in accordance with “Guide for the Care and Use of Laboratory Animals” published by the US National Institute of Health.
Oral glucose tolerance test
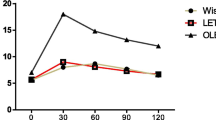
Following a 16-hr overnight fasting period, glucose 2 g·kg−1 was administered to each rat by gavage. Tail blood was sampled before and 30, 60, 90 and 120 min after glucose administration so that blood glucose levels could be measured using a FreeStyle glucometer (TheraSense, Inc., Alameda, CA, USA). The diagnosis of DM was made when the rats met the criterion of an elevated peak glucose level (> 300 mg·dL−1 as well as sustained hyperglycemia) defined by a glucose level > 200 mg·dL−1 at the 120-min time point. Otsuka Long-Evans Fatty rats that failed to meet the criterion were not used for the subsequent experiments. Long-Evans Tokushima Otsuka rats whose plasma glucose level was < 200 mg·dL−1 at 120 min and whose peak glucose level was < 300 mg·dL−1 at any time were considered as non-DM and used for the subsequent experiments.
Measurement of isometric force
Otsuka Long-Evans Fatty and LETO rats were anesthetized with halothane and exsanguinated by cutting through the common carotid artery. The chest of each rat was opened and the descending portion of the thoracic aorta was isolated. The aorta was cleared of excess fat and connective tissue and then was cut into rings 3-4 mm in length. Four to six rings were typically harvested from each rat. The endothelium was removed by gently rubbing the luminal surface with a stainless steel needle. The aortic rings were fixed vertically between hooks in 10-mL organ baths containing Krebs bicarbonate solution (KBS). The constituents of KBS included: NaCl 118.2 mM, KCl 4.8 mM, CaCl2 2.5 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 24.8 mM, and dextrose 10 mM. The solution was bubbled continuously with a mixture of 95% O2 and 5% CO2 to maintain the pH within the range of 7.35-7.45, and the temperature of the solution was kept at 37°C. The hook anchoring the upper end of the rings was connected to the lever of a force displacement transducer (NEC San-ei Instruments, Tokyo, Japan), and the lower hook was fixed to the bottom of the organ bath. Changes in isometric force development were amplified and displayed on chart recorders (Recti-Horiz-8 K, NEC San-ei Instruments, Tokyo, Japan). The resting tension was adjusted to 3.0 g, which was found in our previous studies to be optimal for inducing maximal contraction.6–9 The rings were allowed to equilibrate for 60 min before the start of the experiments, during which time the bathing solution was replaced every 15 min.
After equilibration, the aortic rings were incubated with KCl 30 mM to assess their overall contractile responsiveness. A lack of the relaxation response to acetylcholine 10−6 M in rings precontracted with phenylephrine 3 × 10−7 M confirmed removal of the endothelium. Only aortic rings that developed contractile force of at least 1.0 g in response to KCl 30 mM and exerted no relaxation response to acetylcholine were used for subsequent experiments. Sevoflurane was introduced into the gas mixture through agent-specific vaporizers (Penlon, Abingdon, Oxon, UK). The concentrations in the resulting gas mixture were monitored and adjusted using a calibrated Atom 303 anesthetic agent monitor (Atom, Tokyo, Japan). The concentrations of the sevoflurane in the bathing solution, as measured by gas chromatography (Shimadzu CO, Kyoto, Japan), were the same as the concentrations of sevoflurane in our study,6 which used the same experimental system (0.17 mM, 95% CI 0.14-0.20 mM; 0.35 mM, 95% CI 0.34-0.36 mM; and 0.50 mM, 95% CI 0.48-0.52 mM) at sevoflurane concentrations of 1.7%, 3.4%, and 5.1%, respectively (n = 8-12).
Norepinephrine 10−6 M was used to induce vascular contraction, based on the cumulative concentration – response relationships of the rings to NE 10−11 -10−6 M determined in a preliminary investigation. To assess the effects of sevoflurane on NE-induced contraction, three aortic rings from each of five different rats were exposed randomly to 0, 1.7, or 3.4% sevoflurane for 15 min before NE challenge (n = 5). Each ring was exposed to only one concentration of sevoflurane, and rings from a single rat were not exposed to the same concentration of sevoflurane. Isometric force development in response to NE was expressed as a percentage relative to that induced by KCl 30 mM.
[Ca2+]i measurement
Endothelium-denuded aortic strips, approximately 5 mm long and 3.5 mm wide, were prepared from the isolated rat descending thoracic aorta. Two to three strips were typically harvested from each rat. The strips were incubated for six hours in KBS containing the acetoxymethyl ester of fura-2 10−5 M at room temperature (20°C to 22°C). A noncytotoxic detergent, cremophor 0.1%, was added to the solution to increase the solubility of the acetoxymethyl ester. After the loading period, the preparations were washed three times with KBS. Each aortic strip was held horizontally in a temperature-controlled (37°C) organ bath that was perfused continuously with KBS aerated with a mixture of 95% O2 and 5% CO2. Fluorescence measurements were performed using a dual-wavelength spectrofluorometer (CAF-110, Japan Spectroscopic, Tokyo, Japan) at excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm. The 340 to 380 nm fluorescence ratio was used as an indicator of [Ca2+]i. The change in the 340/380 ratio in response to KCl 30 mM was initially observed, and this value was used as a standard of reference (100%). After washing with bathing solution, sevoflurane at concentrations of 0, 1.7, or 3.4% was introduced into the aerated gas mixture for 15 min, followed by the addition of NE 10−6 M to the bathing solution. The changes in the 340/380 ratio were recorded and expressed relative to the reference value.
Materials and drugs
Norepinephrine was purchased from Sigma-Aldrich Fine Chemicals (St. Louis, MO, USA). The stock solution of NE was stabilized with L- (+) ascorbic acid 10−6M, and the final concentration of ascorbic acid in the organ bath was < 10−7M. Sevoflurane was obtained from Dinabot Co. (Osaka, Japan). The acetoxymethyl ester of fura-2 solution was obtained from Dojindo Laboratories (Kumamoto, Japan).
Data analysis
All data are presented as mean (95% CI). The sample size (n) represents the number of animals from which the aortic rings and strips were taken. One-way analysis of variance (ANOVA) with Games-Howell-test for post hoc comparison was performed to compare the effects of different concentrations of sevoflurane on the NE-induced contraction and [Ca2+]i using SPSS for Windows version 16.0 (SPSS Japan Inc., Tokyo, Japan).10 Interaction between rat type and sevoflurane concentration was evaluated by two-way ANOVA. All P values were two-tailed and P < 0.05 was considered as statistically significant.
Result
More than 90% of the OLETF rats were diagnosed as DM, and almost all LETO rats were diagnosed as non-DM. The Table shows the body weights and the results of the Oral Glucose Tolerance Test (OGTT) in OLETF and age-matched LETO rats used in this study. The body weights of the OLETF rats were greater than those of the LETO rats. The increases in plasma glucose in response to oral glucose were greater in the OLETF rats compared with the LETO rats (Table 1).
Both KCl 30 mM and NE 10−6 M elicited rapid and sustained increases in tension and [Ca2+]i in rat aortic smooth muscle (Fig. 1). The KCl-induced contractions in LETO and OLETF rats were 1.6 g, 95% CI 1.1-2.1 g and 1.7 g, 95% CI 1.0-2.4 g, respectively (n = 5, each). The NE-induced contraction in the LETO and OLETF rats reached the maximum level of 46.4%, 95% CI 39.0-53.7% and 54.8%, 95% CI 36.9-72.6%, respectively, of the KCl-induced contraction (n = 5, each). There were no significant differences in the contractile responses to KCl (P = 0.82) and NE (P = 0.46) between the LETO and the OLETF rats (Fig. 2).
Sevoflurane could inhibit the contractile response to NE in a concentration-dependent manner in LETO rats but not in OLETF rats (Fig. 3). The inhibitory effect of 3.4% sevoflurane on contraction differed significantly between the LETO and the OLETF rats (P = 0.022). Two-way ANOVA revealed a significant interaction between rat type and sevoflurane concentration (P = 0.020). Similarly, exposure to sevoflurane also attenuated the increase of [Ca2+]i in response to NE in LETO rats but had no effect on [Ca2+]i in response to NE in OLETF rats (n = 5, each) (Fig. 4). The inhibitory effect of sevoflurane on the change in [Ca2+]i was greater in LETO rats than in OLETF rats. (P = 0.022 at 1.7%; P < 0.001 at 3.4%). The interaction between rat type and the sevoflurane concentration of [Ca2+]i in response to NE was also significant (P = 0.002).
The effects of sevoflurane on norepinephrine (NE 10−6 M)-induced contraction of aortic smooth muscle in Long Evans Tokushima Otsuka (LETO) rats and Otsuka Long-Evans Tokushima Fatty (OLETF) rats (n = 5). In LETO rats, sevoflurane inhibited the contraction in response to NE in a concentration-dependent manner, but sevoflurane had no effect in OLETF rats. Changes in tension are expressed relative to those induced by KCl 30 mM. Two-way analysis of variance revealed a significant interaction between rat type and sevoflurane concentration (P = 0.020)
The effects of sevoflurane on norepinephrine (NE 10−6M)-induced elevation of intracellular Ca2+ concentration ([Ca2+]i) of aortic smooth muscle in Long Evans Tokushima Otsuka (LETO) rats and Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Sevoflurane did not affect the increase in [Ca2+]i (n = 5) in response to NE in OLETF rats, but it inhibited the response in LETO rats. Changes in [Ca2+]i are expressed relative to those induced by KCl 30 mM. Two-way analysis of variance revealed a significant interaction between rat type and sevoflurane concentration (P = 0.002)
Discussion
Following are the key findings of the current study. The extent of NE-induced vascular contraction and increases in [Ca2+]i observed in the OLETF rats were similar to those in the LETO rats. In the LETO rats, sevoflurane attenuated the NE-induced contractile response and increase in [Ca2+]i in a concentration-dependent manner. By contrast, sevoflurane did not alter the NE-induced contractile response and increase in [Ca2+]i in OLETF rats.
Although endothelial dysfunction is an important early step in the development of atherosclerosis in diabetic patients,11 abnormal responses of the underlying vascular smooth muscle to various neurohumoral factors have also been demonstrated. The responsiveness of arteries from diabetic animals remains controversial, i.e., decreased,12 increased,13 or unchanged14 responses to NE have been reported. Almost all of these studies were conducted using experimental animals treated with diabetogenic drugs, such as STZ, that destroys the beta cell in the pancreas due to its alkylating properties and subsequently depletes the insulin secretion.15 Therefore, STZ-induced DM is considered as a model of type 1 DM. By contrast, OLETF rats spontaneously develop hyperinsulinemia from 25-60 weeks of age and have been characterized as a genetic model of type 2 DM.16 The presence of hyperinsulinemia is considered as the major difference between type 1 and type 2 DM, and long-term hyperinsulinemia has been shown to cause the changes in vascular reactivity.17 Even in STZ-treated rats, increased vascular contractility has been observed in an insulin-dependent STZ DM model in contrast to decreased contractility in an insulin-independent STZ DM model,18 suggesting that the diverse vasoreactivity seen in the STZ-induced DM rats might be explained by differences in the duration or extent of their diabetic status. Although the mechanism underlying the vascular contraction in type 2 DM might be different from that in type 1 DM, no reports have clearly demonstrated the differences in vasoconstriction between type 1 and type 2 DM. Some reports have shown the increased vascular contractile response in type 2 DM.19–21 In the present study, similar contractile effects and increases in [Ca2+]i in response to NE were observed in both strains of rats. The discrepancy between our findings and those from the previous studies may be due to the concentration of NE tested. We used the highest concentration of NE (10−6 M) because the concomitant assessment of calcium mobilization requires a high concentration. It has been reported that the maximum contractile response to a high concentration of NE in DM rats is similar to that in non-DM rats.22
A body of evidence has accumulated regarding the effects of volatile anesthetics, including sevoflurane, on vascular contraction, and most of these studies have demonstrated inhibitory effects of volatile anesthetics on agonist-induced vascular contraction and increases in [Ca2+]i in various types of arteries.23 However, no information is available regarding the effects of volatile anesthetics on the contraction and Ca2+ mobilization of the vessels from diabetic models. Sevoflurane has been shown to attenuate Ca2+ influx from the extracellular space, Ca2+-induced Ca2+ release, and to stimulate the inositol 1,4,5-triphosphate (IP3)-induced Ca2+ release in non-DM vascular contraction.24 Augmentation of Ca2+ influx through a voltage-dependent Ca2+ channel25 and IP3- mediated Ca2+ release26 has also been reported in vascular smooth muscle from type 2 DM. While it might be possible that sevoflurane exerts a contradictory effect on Ca2+ transients, the inhibition of Ca2+ influx and potentiation of IP3-induced Ca2+ release would counteract with each other and result in no change in [Ca2+]i. The intracellular mechanism by which sevoflurane fails to affect Ca2+ transients and vascular contraction in response to NE remain unclear, since the measurement of [Ca2+]i using tissue preparation cannot distinguish between Ca2+ influx and the intracellular release of Ca2+. Consistent with the present findings, we have demonstrated that anesthetic induction with sevoflurane increases peripheral skin temperature in non-DM patients, but not in type 2 DM patients, indicating a lack of vasodilating effect of sevoflurane in peripheral skin vessels of DM patients.5 Further study will be needed to elucidate the exact mechanism(s) underlying the effect of anesthetics on diabetic vessels.
The major limitation of the current in vitro study is as follows. First, we used aortic preparations, which are conduit vessels and may exhibit different reactivity compared with the small arteries that determine the peripheral vascular resistance. Thus, the data obtained from large conduit arteries may not be extrapolated directly to the in vivo situation; although our previous clinical study indirectly suggested a similar response to sevoflurane in peripheral skin vessels in diabetic patients.5 Secondly, vascular contraction is well known to be mediated by both changes in [Ca2+]i and myofilament Ca2+ sensitivity. We assessed only [Ca2+]i in this study and did not evaluate myofilament Ca2+-sensitivity. Volatile anesthetics have multiple intracellular sites of action.27 We have recently reported that sevoflurane inhibited the phosphorylation of protein kinase C (PKC) and PKC-potentiated inhibitory protein (CPI-17)28 in response to angiotensin II. Selective enhancement of the NE-induced activation of the PKC/CPI-17 pathway has been postulated in arteries from diabetic rats.29 It is possible that the mechanisms involving myofilament Ca2+-sensitivity could influence the results.
In summary, the current study demonstrated that NE caused contraction and increases in [Ca2+]i of aortic smooth muscle from type 2 diabetic rats to the same extent as those from non-diabetic control rats. These responses to NE were inhibited significantly by clinical concentrations of sevoflurane in non-DM rats, but not in diabetic rats. Thus the mechanism(s) of vasodilation of vascular smooth muscle in response to sevoflurane may be altered in DM.
References
Jermendy G. Clinical consequences of cardiovascular autonomic neuropathy in diabetic patients. Acta Diabetol 2003; 40(Suppl 2): S370-4.
Giorda CB, Avogaro A, Maggini M, et al. Incidence and risk factors for stroke in type 2 diabetic patients: the DAI study. Stroke. 2007; 38: 1154-60.
Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 1998; 47: 859-66.
Pierce GN, Maddaford TG, Russell JC. Cardiovascular dysfunction in insulin-dependent and non-insulin-dependent animal models of diabetes mellitus. Can J Physiol Pharmacol 1997; 75: 343-50.
Negoro T, Mizumoto K, Ogawa K, Hironaka Y, Kakutani T, Hatano Y. Effects of isoflurane and sevoflurane anesthesia on arteriovenous shunt flow in the lower limb of diabetic patients without autonomic neuropathy. Anesthesiology 2007; 107: 45-52.
Yu J, Ogawa K, Tokinaga Y, Iwahashi S, Hatano Y. The vascular relaxing effects of sevoflurane and isoflurane are more important in hypertensive than normotensive rats. Can J Anesth 2004; 51: 979-85.
Yu J, Tokinaga Y, Ogawa K, Iwahashi S, Hatano Y. Sevoflurane inhibits angiotensin II-induced, protein kinase C-mediated but not Ca2+-elicited contraction of rat aortic smooth muscle. Anesthesiology 2004; 100: 879-84.
Yu J, Ogawa K, Tokinaga Y, Hatano Y. Sevoflurane inhibits guanosine 5’-[gamma-thio] triphosphate-stimulated, Rho/Rho-kinase-mediated contraction of isolated rat aortic smooth muscle. Anesthesiology 2003; 99: 646-51.
Yu J, Mizumoto K, Tokinaga Y, Ogawa K, Hatano Y. The inhibitory effects of sevoflurane on angiotensin II-induced, p44/42 mitogen-activated protein kinase-mediated contraction of rat aortic smooth muscle. Anesth Analg 2005; 101: 315-21.
Ramsey PH, Ramsey PP. Power and type I errors for pairwise comparisons of means in the unequal variances case. Br J Math Stat Psychol 2009; 62(Pt 2): 263-81.
Duncan ER, Walker SJ, Ezzat VA, et al. Accelerated endothelial dysfunction in mild prediabetic insulin resistance: the early role of reactive oxygen species. Am J Physiol Endocrinol Metab 2007; 293: E1311-9.
Cameron NE, Cotter MA. Impaired contraction and relaxation in aorta from streptozotocin-diabetic rats: role of polyol pathway. Diabetologia 1992; 35: 1011-9.
Taylor PD, Oon BB, Thomas CR, Poston L. Prevention by insulin treatment of endothelial dysfunction but not enhanced noradrenaline-induced contractility in mesenteric resistance arteries from streptozotocin-induced diabetic rats. Br J Pharmacol 1994; 111: 35-41.
Fulton DJ, Hodgson WC, Sikorski BW, King RG. Attenuated responses to endothelin-1, KCl and CaCl2, but not noradrenaline, of aortae from rats with streptozotocin-induced diabetes mellitus. Br J Pharmacol 1991; 104: 928-32.
Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008; 51: 216-26.
Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract 1994; 24: S317-20.
Russell JC. Reduction and prevention of the cardiovascular sequelae of the insulin resistance syndrome. Curr Drug Targets Cardiovasc Haematol Disord 2001; 1: 107-20.
Nobe K, Sakai Y, Maruyama Y, Momose K. Hyper-reactivity of diacylglycerol kinase is involved in the dysfunction of aortic smooth muscle contractility in streptozotocin-induced diabetic rats. Br J Pharmacol 2002; 136: 441-51.
Brondum E, Kold-Petersen H, Nilsson H, Flyvbjerg A, Aalkjaer C. Increased contractility to noradrenaline and normal endothelial function in mesenteric small arteries from the Goto-Kakizaki rat model of type 2 diabetes. J Physiol Sci 2008; 58: 333-9.
Winters B, Mo Z, Brooks-Asplund E, et al. Reduction of obesity, as induced by leptin, reverses endothelial dysfunction in obese (Lep(ob)) mice. J Appl Physiol 2000; 89: 2382-90.
Lesniewski LA, Donato AJ, Behnke BJ, et al. Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. Am J Physiol Heart Circ Physiol 2008; 294: H1840-50.
Kobayashi T, Matsumoto T, Ooishi K, Kamata K. Differential expression of alpha2D-adrenoceptor and eNOS in aortas from early and later stages of diabetes in Goto-Kakizaki rats. Am J Physiol Heart Circ Physiol 2004; 287: H135-43.
Akata T, Kanna T, Yoshino J, Takahashi S. Mechanisms of direct inhibitory action of isoflurane on vascular smooth muscle of mesenteric resistance arteries. Anesthesiology 2003; 99: 666-77.
Akata T, Nakashima M, Izumi K. Comparison of volatile anesthetic actions on intracellular calcium stores of vascular smooth muscle: investigation in isolated systemic resistance arteries. Anesthesiology 2001; 94: 840-50.
Yoo HJ, Kozaki K, Akishita M, et al. Augmented Ca2 + influx is involved in the mechanism of enhanced proliferation of cultured vascular smooth muscle cells from spontaneously diabetic Goto-Kakizaki rats. Atherosclerosis 1997; 131: 167-75.
Evans JF, Lee JH, Ragolia L. Ang-II-induced Ca(2 +) influx is mediated by the 1/4/5 subgroup of the transient receptor potential proteins in cultured aortic smooth muscle cells from diabetic Goto-Kakizaki rats. Mol Cell Endocrinol 2009; 302: 49-57.
Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 2: regulatory mechanisms modulating Ca2 + mobilization and/or myofilament Ca2 + sensitivity in vascular smooth muscle cells. J Anesth 2007; 21: 232-42.
Qi F, Ogawa K, Tokinaga Y, Uematsu N, Minonishi T, Hatano Y. Volatile anesthetics inhibit angiotensin II-induced vascular contraction by modulating myosin light chain phosphatase inhibiting protein, CPI-17 and regulatory subunit, MYPT1 phosphorylation. Anesth Analg 2009; 109: 412-7.
Mueed I, Zhang L, MacLeod KM. Role of the PKC/CPI-17 pathway in enhanced contractile responses of mesenteric arteries from diabetic rats to alpha-adrenoceptor stimulation. Br J Pharmacol 2005; 146: 972-82.
Funding
This work was supported by institutional and departmental sources.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujii, K., Ogawa, K., Tokinaga, Y. et al. Sevoflurane does not alter norepinephrine-induced intracellular Ca2+ changes in the diabetic rat aorta. Can J Anesth/J Can Anesth 57, 1095–1101 (2010). https://doi.org/10.1007/s12630-010-9387-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9387-0