Abstract
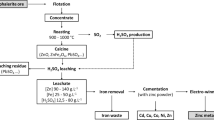
Sulfuric acid leaching of high iron-bearing zinc calcine was investigated to assess the effects of sulfuric acid concentration, liquid- to-solid ratio, leaching time, leaching temperature, and the stirring speed on the leaching rates of zinc and iron. The results showed that the sulfuric acid concentration, liquid-to-solid ratio, leaching time, and leaching temperature strongly influenced the leaching of zinc and iron, whereas stirring speed had little influence. Zinc was mainly leached and the leaching rate of iron was low when the sulfuric acid concentration was less than 100 g/L. At sulfuric acid concentrations higher than 100 g/L, the leaching rate of iron increased quickly with increasing sulfuric acid concentration. This behavior is attributed to iron-bearing minerals such as zinc ferrite in zinc calcine dissolving at high temperatures and high sulfuric acid concentrations but not at low temperatures and low sulfuric acid concentrations.
Similar content being viewed by others
References
A.D. Souza, P.S. Pina, V.A. Leão, C.A. Silva, and P.F Siqueira, The leaching kinetics of a zinc sulphide concentrate in acid ferric sulphate, Hydrometallurgy, 89(2007), No. 1-2, p. 72.
T.T. Chen and J.E. Dutrizac, Mineralogical changes occurring during the fluid-bed roasting of zinc sulfide concentrates, JOM, 56(2004), No. 12, p. 46.
J.C. Balarini, L. de Oliveira Polli, T.L.S. Miranda, R.M.Z. de Castro, and A. Salum, Importance of roasted sulphide concentrates characterization in the hydrometallurgical extraction of zinc, Miner. Eng., 21(2008), No. 1, p. 100.
B. Boyanov, A. Peltekov, and V. Petkova, Thermal behavior of zinc sulfide concentrates with different iron content at oxidative roasting, Thermochim. Acta, 586(2014), No. 8, p. 9.
N. Leclerc, E. Meux, and J.M. Lecuire, Hydrometallurgical extraction of zinc from zinc ferrites, Hydrometallurgy, 70(2003), No. 1-3, p. 175.
K. Wantae and S. Fumio, Mechanochemical synthesis of zinc ferrite from zinc oxide and α-Fe2O3, Powder Technol., 114(2001), No. 1-3, p. 12.
K. Brunelli and M. Dabalà, Ultrasound effects on zinc recovery from EAF dust by sulfuric acid leaching, Int. J. Miner. Metall. Mater., 22(2015), No. 4, p. 353.
M.B. Pavlović, Č. Jovalekić, A.S. Nikolić, D. Manojlović, and N. Šojić, Soft mechanochemical synthesis of MgFe2O4 nanoparticles from the mixture of α-Fe2O3 with Mg(OH)2 and Fe(OH)3 with Mg(OH)2, Mater. Sci. Technol., 26(2010), No. 8, p. 968.
K. Li, C. Peng, and K. Jiang, The recycling of Mn–Zn ferrite wastes through a hydrometallurgical route, J. Hazard. Mater., 194(2011), No. 5, p. 79.
S. Oleszek-Kudlak, M. Grabda, and T. Nakamura, Alternative method for pyrometallurgical recycling of EAF dust using plastic waste containing tetrabromobisphenol A, High Temp. Mater. Processes, 30(2011), No. 4-5, p. 359.
H.G. Wang, Y. Li, J.M. Gao, M. Zhang, and M. Guo, A novel hydrothermal method for zinc extraction and separation from zinc ferrite and electric arc furnace dust, Int. J. Miner. Metall. Mater., 23(2016), No. 2, p. 146.
K. Nowińska, Z. Adamczyk, and E. Melaniuk-Wolny, Accompanying elements in sphalerite in pyrometallurgical process of zinc and lead production, Mater. Manuf. Processes, 30(2015), No. 12, p. 1457.
H. Yan, L.Y. Chai, B. Peng, M. Li, N. Peng, and D.K. Hou, A novel method to recover zinc and iron from zinc leaching residue, Miner. Eng., 55(2014), p. 103.
B. Janković, S. Stopić, A. Güven, and B. Friedrich, Kinetic modeling of thermal decomposition of zinc ferrite from neutral leach residues based on stochastic geometric model, J. Magn. Magn. Mater., 358-359(2014), No. 5, p. 105.
C.C. Wu, F.C. Chang, W.S. Chen, M.S. Tsai, and Y.N. Wang, Reduction behavior of zinc ferrite in EAF-dust recycling with CO gas as a reducing agent, J. Environ. Manage., 143(2014), No. 10, p. 208.
G. Yu, N. Peng, L. Zhou, Y.J. Liang, X.Y. Zhou, B. Peng, L.Y. Chai, and Z.H. Yang, Selective reduction process of zinc ferrite and its application in treatment of zinc leaching residues, Trans. Nonferrous Met. Soc. China, 25(2015), No. 8, p. 2744.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51774099 and 51364003) and the Ministry-Province Jointly Constructed Cultivation Base for State Key Laboratory of Processing for Non-ferrous Metal and Featured Materials, Guangxi Zhuang Autonomous Region, China (GXKFJ16-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Jl., Liu, Jg., Xiao, Hx. et al. Sulfuric acid leaching of high iron-bearing zinc calcine. Int J Miner Metall Mater 24, 1211–1216 (2017). https://doi.org/10.1007/s12613-017-1513-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-017-1513-3