Abstract
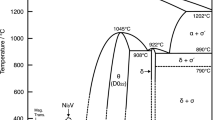
Interdiffusion in the Fe2O3-TiO2 system was investigated by the diffusion couple method in the temperature range of 1323 to 1473 K. The diffusion concentration curves of Ti4+ cations were obtained by electron probe microanalysis, according to which the Boltzmann-Matano method optimized by Broeder was used to calculate the interdiffusion coefficients. The interdiffusion coefficients almost increased linearly with the mole fraction of Ti4+ cations increasing, and they were in the range of 10−12–10−11cm2·s−1. The increase of temperature could also lead to the increase of the interdiffusion coefficients at a constant concentration of Ti4+ cations. It was also found that the thickness growth of the diffusion layer obeyed the parabolic rate law.
Similar content being viewed by others
References
D.S. Ginley and R.J. Baughman, Preparation and czochralski crystal growth of the iron titanates, FeTiO3, Fe2TiO4, and Fe2TiO5, Mater. Res. Bull., 11(1976), No. 12, p. 1539.
M.H. Khedr, M. Bahgat, M. Radwan, and H.S. Abdelmaksoud, Effect of Cu2+ on the magnetic properties and reducibility of Fe2TiO5, J. Mater. Process. Technol., 190(2007), No. 1–3, p. 153.
A.R. Phani, F. Ruggieri, M. Passacantando, and S. Santucci, Low temperature growth of nanocrystalline Fe2TiO5 perovskite thin films by sol-gel process assisted by microwave irradiation, Ceram. Int., 34(2008), p. 205.
B. Pal, M. Sharonb, and G. Nogami, Preparation and characterization of TiO2/Fe2O3 binary mixed oxides and its photocatalytic properties, Mater. Chem. Phys., 59(1999), p. 254.
G.R. Xu and C.J. Li, Fabrication of Fe/Co-Ti-O nanofibers: photocatalytic activity and magnetic properties, Environ. Chem., 30(2011), No. 3, p. 616.
K. Hirota and R.C. Bradt, Sintering and synthesis of the pseudobrookite oxide (Fe2TiO5) by the solid state reaction, Anal. Sci., 7(1991), Suppl., p. 1275.
H. Kozuka and M. Kajimura, Sol-gel preparation and photoelectrochemical properties of Fe2TiO5 thin films, J. Sol Gel Sci. Technol., 22(2001), p. 125.
A.R. Phani and S. Santucci, Structural characterization of iron titanium oxide synthesized by sol-gel spin-coating technique, Mater. Lett., 50(2001), p. 240.
C. Matano, On the relation between the diffusioncoefficients and concentrations of solid metals (the nickelcopper system), Jpn. J. Phys., 8(1933), No. 3, p. 109.
M. Palcut, R. Knibbe, K. Wiik, and T. Grande, Cation inter-diffusion between LaMnO3 and LaCoO3 materials, Solid State Ionics, 202(2011), p. 6.
H. Fukuyama, Md.K. Hossain, and K. Nagata, Solid-state reaction kinetics of the system Cao-FeO, Metall. Mater. Trans. B, 33(2002), p. 257.
F.J.A. den Broeder, A general simplification and improvement of the Matano-Boltzmann method in the determination of the interdiffusion coefficients in binary systems, Scrpta Metall., 3(1969), p. 321.
C. Greskovich and V.S. Stubican, Interdiffusion studies in the system MgO-Cr2O3, J. Phys. Chem. Solids, 30(1969), p. 909.
P. Zhang, T. Debroy, and S. Seetharaman, Interdiffusion in the MgO-Al2O3 spinel with or without some dopants, Metall. Mater. Trans. A, 27(1996), p. 2105.
Y.M. Sung, W.C. Kwak, and S. Kim, Kinetics of PbTiO3 perovskite phase formation via an interfacial reaction, J. Mater. Res., 17(2002), No. 2, p. 407.
R.F. Davis and J.A. Pask, Diffusion and reaction studies in the system Al2O3-SiO2, J. Am. Ceram. Soc., 55(1972), No. 10, p. 525.
M. O’keeffe and T.J. Ribble, Interdiffusion and the solubility limits of Cr2O3 in the rutile phase of TiO2, J. Solid State Chem., 4(1972), p. 351.
A.E. Paladino and W.D. Kingery, Aluminum ion diffusion in aluminum oxide, J. Chem. Phys., 37(1962), No. 5, p. 957.
J. Sanchez, D.P. Quinn, and M.C. Tringides, The use of the Boltzmann-Matano analysis in two-dimensional concentration profiles, Surf. Sci., 391(1997), p. 101.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, Zs., Hu, Xj., Li, Sy. et al. Interdiffusion in the Fe2O3-TiO2 system. Int J Miner Metall Mater 20, 273–278 (2013). https://doi.org/10.1007/s12613-013-0723-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-013-0723-6