Abstract
Purpose of Review
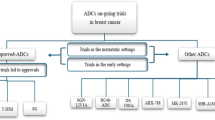
Breast Cancer is estimated to account for approximately 15.5% of all new cancer cases in 2024. While the five-year relative survival rate for women with breast cancer has steadily increased to 91.2% from 2014 to 2020, there remains an unmet need for the treatment of high-risk, early-stage breast cancer and advanced breast cancers. There are a number of ongoing Phase I clinical trials to evaluate the safety, efficacy, pharmacokinetics, and pharmacodynamics of novel therapeutics in breast cancer. This review article aims to provide an overview of early phase therapeutics for patients with advanced breast cancer.
Recent Findings
There are a number of promising early therapeutics being evaluated for the treatment of breast cancer. Encouraging data from numerous ongoing phase I trials evaluating cyclin-dependent kinase inhibitors (CDKi), such as PF-07220060, selective estrogen receptor degraders (SERDs), such as imlunestrant, and antibody-conjugate drug therapies, such as Dato-DXd, have pushed these drugs into further stages of development.
Summary
This article reviews the recently completed and ongoing Phase I clinical trials for novel therapeutics including CDKi therapies, endocrine therapies, and antibody-drug conjugates for the treatment of advanced breast cancer (aBC). Ultimately these novel therapeutics could have an important impact on patients with advanced breast cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data Availability
No datasets were generated or analysed during the current study.
References
Female Breast Cancer. In: Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. 2024. https://seer.cancer.gov/statfacts/html/breast.html. Accessed 14 May 2024.
O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(Suppl 3):20–9. https://doi.org/10.1634/theoncologist.10-90003-20.
Daily K, Douglas E, Romitti PA, Thomas A. Epidemiology of De Novo metastatic breast Cancer. Clin Breast Cancer. 2021;21(4):302–8. https://doi.org/10.1016/j.clbc.2021.01.017.
Chia SK, Speers CH, D’yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110(5):973–9. https://doi.org/10.1002/cncr.22867.
Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–9. https://doi.org/10.1093/annonc/mdn424.
Caswell-Jin JL, Sun LP, Munoz D, et al. Analysis of breast Cancer mortality in the US-1975 to 2019. JAMA. 2024;331(3):233–41. https://doi.org/10.1001/jama.2023.25881.
Altucci L, Addeo R, Cicatiello L, et al. Estrogen induces early and timed activation of cyclin-dependent kinases 4, 5, and 6 and increases cyclin messenger ribonucleic acid expression in rat uterus. Endocrinology. 1997;138(3):978–84. https://doi.org/10.1210/endo.138.3.5002.
Geum D, Sun W, Paik SK, Lee CC, Kim K. Estrogen-induced cyclin D1 and D3 gene expressions during mouse uterine cell proliferation in vivo: differential induction mechanism of cyclin D1 and D3. Mol Reprod Dev. 1997;46(4):450–8. https://doi.org/10.1002/(SICI)1098-2795(199704)46:4<450::AID-MRD2>3.0.CO;2-N.
Wander S, Valacer D, Zuniga R et al. Abstract PO3-18-06: First-in-human phase 1A study of RGT-419B, a next generation CDK4 inhibitor, in patients (pts) with hormone receptor positive (HR+) HER2- advanced/metastatic breast cancer (ABC) who progressed on prior CDK4/6 inhibitors (CDK4/6i). Cancer Res. 2024: 84(9 Suppl):Abstract nr PO3-18-06. https://doi.org/10.1158/1538-7445.SABCS23-PO3-18-06
Xie J, Han J, Zhilong H, et al. Abstract PS16-22:Targeting Resistance to current CDK4/6 therapies by RGT-419B, an inhibitor with an optimized kinase activity spectrum. Cancer Res. 2021;4_Supplement81. https://doi.org/10.1158/1538-7445.SABCS20-PS16-22.
Villalona-Calero M, Mita M, Mita A et al. Abstract B159: A first-in-human study of CDK9 inhibitor KB-0742 demonstrates evidence of tolerability and clinical activity. Mol Cancer Ther 2023:22(12 Suppl):Abstract nr B159. https://doi.org/10.1158/1535-7163.TARG-23-B159
Yap T, Elhaddad A, Grisham R, et al. First-in-human phase 1/2a study of a potent and novel CDK2-selective inhibitor PF-07104091 in patients (pts) with advanced solid tumors, enriched for CDK4/6 inhibitor resistant HR+/HER2- breast cancer. J Clin Oncol. 2023;41:3010–3010. https://doi.org/10.1200/JCO.2023.41.16_suppl.3010.
Yap T, Giordano A, Hamilton EP et al. First-in-human first-in-class phase 1/2a study of the next generation CDK4-selective inhibitor PF-07220060 in patients (pts) with advanced solid tumors, enriched for HR + HER2- mBC who progressed on prior CDK4/6 inhibitors and endocrine therapy. J Clin Oncol. 2023: 41: 3009–3009. doi:10.1200/JCO.2023.41.16_suppl.3009 Encouraging results from this phase I/2a clinical trial during the dose escalation portion showed early efficacy and a tolerable safety profile, which has encouraged initiation of the dose expansion phase of the trial, which will study the drug in combination with endocrine therapy in patients with HR+/HER2 - metastatic breast cancer.
Patel M, Juric D, Henick BS, et al. BLU-222, an oral, potent, and selective CDK2 inhibitor, in patients with advanced solid tumors: phase 1 monotherapy dose escalation. J Clin Oncol. 2023;41:3095–3095. https://doi.org/10.1200/JCO.2023.41.16_suppl.3095.
Akli S, Van Pelt CS, Bui T, Meijer L, Keyomarsi K. Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E. Cancer Res 2011:71(9):3377–86. https://doi.org/10.1158/0008-5472.CAN-10-4086
Juric D, Patel MR, Duska LR, et al. BLU-222, an investigational, oral, potent, and highly selective CDK2 inhibitor (CDK2i), as monotherapy in patients (pts) with advanced solid tumors and in combination with ribociclib (RIBO) and fulvestrant (FUL) in HR+/HER2 – breast cancer (BC). J Clin Oncol. 2024;42:1056–1056. https://doi.org/10.1200/JCO.2024.42.16_suppl.1056.
Acheampong T, Kehm RD, Terry MB, Argov EL, Tehranifar P. Incidence trends of breast Cancer Molecular subtypes by Age and Race/Ethnicity in the US from 2010 to 2016. JAMA Netw Open 2020:3:e2013226. https://doi.org/10.1001/jamanetworkopen.2020.13226
Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140(12):5566–78. https://doi.org/10.1210/endo.140.12.7179.
Smith CL, Nawaz Z, O’Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11(6):657–66. https://doi.org/10.1210/mend.11.6.0009.
Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line treatment of advanced breast Cancer: overall survival analysis from the phase II FIRST Study. J Clin Oncol. 2015;33(32):3781–7. https://doi.org/10.1200/JCO.2015.61.5831.
Ciruelos E, Pascual T, Arroyo Vozmediano ML, et al. The therapeutic role of fulvestrant in the management of patients with hormone receptor-positive breast cancer. Breast. 2014;23(3):201–8. https://doi.org/10.1016/j.breast.2014.01.016.
Jhaveri K, Jeselsohn R, Lim E, et al. A phase 1a/b trial of imlunestrant (LY3484356), an oral selective estrogen receptor degrader (SERD) in ER-positive (ER+) advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): Monotherapy results from EMBER. J Clin Oncol. 2022;40(1021–1022). https://doi.org/10.1200/JCO.2022.40.16_suppl.1021. In patients with HR + advanced breast cancer who are heavily pre-treated, Imlunestrant has shown a tolerable safety profile and promising clinical activity encouraging ongoing phase III trials in both advanced and early breast cancer.
Bidard FC, Kaklamani VG, et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, human epidermal growth factor receptor 2–Negative advanced breast Cancer: results from the Randomized Phase III EMERALD Trial. J Clin Oncol. 2022;40:3246–56. https://doi.org/10.1200/JCO.22.00338.
Rugo H, Bardia A, Cortés J, et al. Abstract PO2-05-04: ELEVATE: a phase 1b/2, open-label, umbrella study evaluating elacestrant in various combinations in patients (pts) with estrogen receptor-positive (ER+), HER2-negative (HER2-) locally advanced or metastatic breast cancer (mBC). Cancer Res. 2024;9_Supplement84. https://doi.org/10.1158/1538-7445.SABCS23-PO2-05-04.
Jhaveri KL, Bellet M, Turner NC, et al. Phase Ia/b study of giredestrant ± palbociclib and ± luteinizing hormone-releasing hormone agonists in Estrogen Receptor-Positive, HER2-Negative, locally Advanced/Metastatic breast Cancer. Clin Cancer Res. 2024;30(4):754–66. https://doi.org/10.1158/1078-0432.CCR-23-1796.
Hamilton WP, Oliviera M, Turner N et al. A phase I dose escalation and expansion study of the next generation oral SERD camizestrant in women with ER-positive, HER2-negative advanced breast cancer: SERENA-1 monotherapy results. Ann Oncol. Published online May 08, 2024. doi: 10.1016/j.annonc.2024.04.01 Promising results in terms of efficacy of this new oral SERD have encouraged the development of phase II and III clinical trials that are attempting to further compare camizestrant to current approved standards of care.
Hamilton E, Vahdat L, Han HS, et al. Abstract PD13-08. First-in-human safety and activity of ARV-471, a novel PROTAC® estrogen receptor degrader, in ER+/HER2- locally advanced or metastatic breast cancer. Cancer Res. 2022;4_Supplement82. https://doi.org/10.1158/1538-7445.SABCS21-PD13-08.
Bhave M, Jhaveri K, Kaufman P, et al. Imlunestrant, an oral selective estrogen receptor degrader (SERD), in combination with human epidermal growth factor receptor 2 (HER2) directed therapy, with or without abemaciclib, in estrogen receptor (ER) positive, HER2 positive advanced breast cancer (aBC): EMBER phase 1a/1b study. J Clin Oncol. 2024;42:1027–1027. https://doi.org/10.1200/JCO.2024.42.16_suppl.1027.
Oliviera M, Hamilton EP, Incorvati J et al. Serena-1: updated analyses from a phase 1 study (parts C/D) of the next-generation oral SERD camizestrant (AZD9833) in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. J Clin Oncol 2022:40: 1032–1032. https://doi.org/10.1200/JCO.2022.40.16_suppl.1032
Oliveira M, Pominchuck D, Nowecki Z, et al. Abstract GS3-02: GS3-02 camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: results of the randomized, multi-dose phase 2 SERENA-2 trial. Cancer Res. 2023;83(5Supplement):GS3–02. https://doi.org/10.1158/1538-7445.sabcs22-gs3-02.
Turner N, Huang-Bartlett C, Kalinsky K, et al. Design of SERENA-6, a phase III switching trial of camizestrant in ESR1-mutant breast cancer during first-line treatment. Future Oncol. 2023;19(8):559–73. https://doi.org/10.2217/fon-2022-1196.
Seock AI, Hamilton EP, Cussac A, et al. SERENA-4: a phase 3 comparison of AZD9833 (camizestrant) plus palbociclib, versus anastrozole plus palbociclib, for patients with ER-positive, HER2-negative advanced breast cancer who have not previously received systemic treatment for advanced disease. J Clin Oncol. 2021;39:TPS1101–1101. https://doi.org/10.1200/JCO.2021.39.15_suppl.TPS1101.
Schott A, Hurvitz S, Ma C, et al. GS3-03 ARV-471, a PROTAC® estrogen receptor (ER) degrader in advanced ER-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer: phase 2 expansion (VERITAC) of a phase 1/2 study. Cancer Res. 2023;83(5 Suppl):AbstractnrGS3–03. https://doi.org/10.1158/1538-7445.SABCS22-GS3-03.
Hamilton E, Jeselsohn R, Hurvitz, et al. Abstract PS15-03: vepdegestrant, a PROteolysis TArgeting chimera (PROTAC) estrogen receptor (ER) degrader, plus palbociclib in ER–positive/human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer: phase 1b cohort. Cancer Res. 2024;84(9Supplement):PS15–03. https://doi.org/10.1158/1538-7445.SABCS23-PS15-03.
Layman RM, Jerzak KJm Hilton JF, et al. TACTIVE-U: Phase 1b/2 umbrella study of ARV-471, a proteolysis targeting chimera (PROTAC) estrogen receptor (ER) degrader, combined with other anticancer treatments in ER + advanced or metastatic breast cancer. J Clin Oncol. 2023;41:TPS1121–TPS1121. https://doi.org/10.1200/JCO.2023.41.16_suppl.TPS1121.
Campone M, Ma CX, De Laurentis M, et al. VERITAC-2: a phase 3 study of vepdegestrant, a PROteolysis TArgeting chimera (PROTAC) estrogen receptor (ER) degrader, vs fulvestrant in ER–positive/human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer. J Clin Oncol. 2023;41(TPS1122–TPS11222023). https://doi.org/10.1200/JCO.2023.41.16_suppl.TPS1122.
A Study of ARV-471 (PF-07850327) Plus Palbociclib Versus Letrozole Plus Palbociclib in Participants With Estrogen Receptor Positive, Human Epidermal Growth Factor Negative Advanced Breast Cancer (VERITAC-3). 2024. https://clinicaltrials.gov/study/NCT05538572. Accessed 14 May 2024.
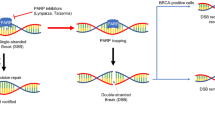
Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. https://doi.org/10.1146/annurev-med-050311-201823.
Mark C, Lee JS, Cui X, Yuan Y. Antibody-drug conjugates in breast Cancer: current status and future directions. Int J Mol Sci. 2023;24(18):13726. https://doi.org/10.3390/ijms241813726.
Bardia A, Krop I, Kogawa T. et a. Datopotamab Deruxtecan in Advanced or Metastatic HR+/HER2– and Triple-Negative Breast Cancer: Results From the Phase I TROPION-PanTumor01 Study. J Clin Oncol 2024: JCO2301909 doi: 10.1200/JCO.23.01909. This abstract presented at San Antonio Breast Cancer demonstrated early efficacy of Dato-DXd in patients that were heavily treated for advanced breast cancer, which has encouraged the development of several phase III trials comparing Dato-DXd to investigator’s choice of chemotherapy in patients with advanced breast cancer.
Krop I, Masuda N, Mukohara T, et al. Patritumab Deruxtecan (HER3-DXd), a human epidermal growth factor receptor 3–Directed antibody-drug Conjugate, in patients with previously treated human epidermal growth factor receptor 3–Expressing metastatic breast Cancer: a Multicenter, Phase I/II Trial. J Clin Oncol. 2023;5550–60. https://doi.org/10.1200/JCO.23.00882.
Cristea MC, Stewart D, Synold T, et al. A phase I study of Mirvetuximab Soravtansine and gemcitabine in patients with FRα-positive recurrent ovarian, primary peritoneal, fallopian tube, or endometrial cancer, or triple negative breast cancer. Gynecol Oncol. 2024;182:124–31. https://doi.org/10.1016/j.ygyno.2023.12.017.
Bardia A, Jhaveri K, Kalinsky K, et al. TROPION-Breast01: Datopotamab Deruxtecan vs chemotherapy in pre-treated inoperable or metastatic HR+/HER2- breast cancer. Future Oncol. 2024;20(8):423–36. https://doi.org/10.2217/fon-2023-0188.
Dent RA, Cescon DW, Bachelot T, et al. TROPION-Breast02: Datopotamab deruxtecan for locally recurrent inoperable or metastatic triple-negative breast cancer. Future Oncol. 2023;19(35):2349–59. https://doi.org/10.2217/fon-2023-0228.
A Study of Dato-DXd With or Without Durvalumab Versus Investigator’s Choice of Therapy in Patients With Stage I-III Triple-negative Breast Cancer Without Pathological Complete Response Following Neoadjuvant Therapy (TROPION-Breast03). 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05629585. Accessed 14 May 2024.
Phase A III. Randomised Study to Evaluate Dato-DXd and Durvalumab for Neoadjuvant/Adjuvant Treatment of Triple-Negative or Hormone Receptor-low/HER2-negative Breast Cancer 2024. https://classic.clinicaltrials.gov/ct2/show/NCT06112379. Accessed 14 May 2024.
Pistilli B, Ibrahimi N, Lacroix-Triki M, et al. 189O A phase II study of patritumab deruxtecan (HER3-DXd), in patients (pts) with advanced breast cancer (ABC), with biomarker analysis to characterize response to therapy (ICARUS-BREAST01). ESMO Open. 2023;8(1):101378. https://doi.org/10.1016/j.esmoop.2023.101378.
Hamilton EP, Dosunmu O, Shastry M et al. A phase 2 study of HER3-DXd in patients with metastatic breast cancer (MBC). J Clin Oncol 2023:41:16. https://doi.org/10.1200/JCO.2023.41.16_suppl.1004
Call JA, Anderson I, Winer I, Orr D, et al. 708 a phase 1/2 study of rinatabart sesutecan (PRO1184), a novel folate receptor alpha-directed antibody-drug conjugate, in patients with locally advanced and/or metastatic solid tumors. J Immunother Cancer. 2023;11(Suppl 1):A1–1731. https://doi.org/10.1136/jitc-2023-SITC2023.0708.
Funding
There is no funding for this review.
Author information
Authors and Affiliations
Contributions
S.V was primary author and wrote the main manuscript text. M.B wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Competing Interests
Dr. Vemuru has no conflict of interest. Dr. Bhave reports consulting fees from Eli Lilly, Gilead, Merck, Astrazeneca, Daiichi Sankyo, and Pfizer, outside the submitted work. Dr. Kalinsky reports grants from Genentech/Roche, Immunomedics, Seattle Genetics, Daiichi Sankyo, Puma Biotechnology, Mersana, Menarini Silicon Biosystems, Myovant Sciences, Takeda, and personal fees from EQRX, ADC Therapeutics (Spouse: Employee), during the conduct of the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vemuru, S., Kalinsky, K. & Bhave, M. Novel Therapeutics in Advanced Breast Cancer: A Review of Current Phase I Clinical Trials. Curr Breast Cancer Rep 17, 2 (2025). https://doi.org/10.1007/s12609-024-00564-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s12609-024-00564-z