Abstract
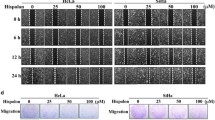
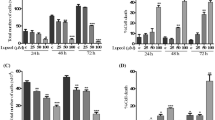
Cyclic lipopeptides secreted by the probiotic bacterium Bacillus subtilis have attracted much attention due to their antitumor activities and low toxicity. However, the role of Mycosubtilin (Myco) in the prevention and treatment of cervical cancer remains unclear. In the present study, we conducted a systematic evaluation of Myco’s anti-cervical cancer effects to identify its molecular mechanism of action using proteomics technology. The results reveal that Myco inhibited the growth of HeLa and SiHa cervical cancer cell lines in a dose-dependent (3–15 µg/mL) and time-dependent (12–48 h) manner and significantly reduced colony formation and migration in HeLa cells, highlighting its potential to suppress tumor spread. Moreover, autophagosome and autolysosome numbers were significantly increased after Myco treatment, and the expression of autophagy-related proteins was significantly modulated, suggesting that autophagy plays a role in its anti-cancer mechanism. Myco treatment also induced G1 phase cell cycle arrest in HeLa cells, as confirmed by proteomics analysis. Myco was shown to induce cell cycle arrest in HeLa cells by regulating the P53 pathway and autophagy-dependent cell death via the PI3K/AKT/mTOR signaling pathway, demonstrating its multidimensional effect on cervical cancer cell growths. Myco treatment significantly inhibited tumor growth in vivo in a nude mouse cervical cancer xenograft model, providing direct evidence of its potential as a therapeutic candidate for cervical cancer. Given its unique anti-cancer mechanism and significant therapeutic efficacy, Myco should be considered a promising therapeutic agent for cervical cancer.







Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- Myco:
-
Mycosubtilin
- DMSO:
-
Dimethyl sulfoxide
- HPLC:
-
High-performance liquid chromatography
- DMEM:
-
Dulbecco’s modified eagle medium
- RPMI:
-
Roswell park memorial institute
- CCK- 8:
-
Cell counting kit-8
- G1:
-
First gap
- S:
-
Synthesis
- G2:
-
Second gap
- M:
-
Metaphase
- BS-Z15:
-
Bacillus subtilis-Z15
- H&E:
-
Hematoxylin and eosin
- OD:
-
Optical density
- PBS:
-
Phosphate-buffered saline
- PI:
-
Propidium iodide
- RNase A:
-
Ribonuclease A
- MDC:
-
Monodansulfonyl cadaverine
- 3-MA:
-
3-Methyladenine
- RIAP:
-
Radio immunoprecipitation assay
- BCA:
-
Bicinchoninic acid
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- PVDF:
-
Polyvinylidene fluoride
- TBST:
-
Tris-buffered saline and Tween 20
- ECL:
-
Enhanced chemiluminescence
- IC50 :
-
Half maximal inhibitory concentration
- DDP:
-
Diamminedichloroplatinum
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
References
Lei J, Arroyo-Mühr LS, Lagheden C et al (2022) Human papillomavirus infection determines prognosis in cervical cancer. J Clin Oncol 40:1522–1528. https://doi.org/10.1200/JCO.21.01930
Marret G, Borcoman E, Tourneau CL (2019) Pembrolizumab for the treatment of cervical cancer. Expert Opinion on Biological Therapy
Liu Y, Li T, Guo R et al (2023) The vaginal microbiota among the different status of human papillomavirus infection and bacterial vaginosis. J Med Virol 95:e28595. https://doi.org/10.1002/jmv.28595
Florea A-M, Busselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 3:1351–1371. https://doi.org/10.3390/cancers3011351
Oun R, Moussa YE, Wheate NJ (2018) The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans 47:6645–6653. https://doi.org/10.1039/c8dt00838h
Fu R, Zhao B, Chen M et al (2023) Moving beyond cisplatin resistance: mechanisms, challenges, and prospects for overcoming recurrence in clinical cancer therapy. Med Oncol 41:9. https://doi.org/10.1007/s12032-023-02237-w
Makovec T (2019) Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol 53:148–158. https://doi.org/10.2478/raon-2019-0018
Xia Y, Sun M, Huang H, Jin W-L (2024) Drug repurposing for cancer therapy. Signal Transduct Target Ther 9:92. https://doi.org/10.1038/s41392-024-01808-1
Qin R, You F-M, Zhao Q et al (2022) Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: from molecular mechanisms to potential therapeutic targets. J Hematol Oncol 15:133. https://doi.org/10.1186/s13045-022-01350-z
Zhou Q, Meng Y, Li D et al (2024) Ferroptosis in cancer: from molecular mechanisms to therapeutic strategies. Signal Transduct Target Ther 9:55. https://doi.org/10.1038/s41392-024-01769-5
De Martino M, Rathmell JC, Galluzzi L, Vanpouille-Box C (2024) Cancer cell metabolism and antitumour immunity. Nat Rev Immunol 24:654–669. https://doi.org/10.1038/s41577-024-01026-4
Deng W, Shang H, Tong Y et al (2024) The application of nanoparticles-based ferroptosis, pyroptosis and autophagy in cancer immunotherapy. Journal of Nanobiotechnology 22:97. https://doi.org/10.1186/s12951-024-02297-8
Debnath J, Gammoh N, Ryan KM (2023) Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol 24:560–575. https://doi.org/10.1038/s41580-023-00585-z
Liu J, Wu Y, Meng S et al (2024) Selective autophagy in cancer: mechanisms, therapeutic implications, and future perspectives. Mol Cancer 23:22. https://doi.org/10.1186/s12943-024-01934-y
Mafi S, Ahmadi E, Meehan E et al (2023) The mTOR signaling pathway interacts with the ER stress response and the unfolded protein response in cancer. Cancer Res 83:2450–2460. https://doi.org/10.1158/0008-5472.CAN-22-3032
Matthews HK, Bertoli C, de Bruin RAM (2022) Cell cycle control in cancer. Nat Rev Mol Cell Biol 23:74–88. https://doi.org/10.1038/s41580-021-00404-3
Diaz-Moralli S, Tarrado-Castellarnau M, Miranda A, Cascante M (2013) Targeting cell cycle regulation in cancer therapy. Pharmacol Ther 138:255–271. https://doi.org/10.1016/j.pharmthera.2013.01.011
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES (2015) The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 14:130–146. https://doi.org/10.1038/nrd4504
Otto T, Sicinski P (2017) Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer 17:93–115. https://doi.org/10.1038/nrc.2016.138
Ramírez-Rendon D, Passari AK, Ruiz-Villafán B et al (2022) Impact of novel microbial secondary metabolites on the pharma industry. Appl Microbiol Biotechnol 106:1855–1878. https://doi.org/10.1007/s00253-022-11821-5
Théatre A, Hoste ACR, Rigolet A et al (2022) Bacillus sp.: a remarkable source of bioactive lipopeptides. Adv Biochem Eng Biotechnol 181:123–179. https://doi.org/10.1007/10_2021_182
Zhang Q, Lin R, Yang J et al (2023) Transcriptome analysis reveals that C17 mycosubtilin antagonizes Verticillium dahliae by interfering with multiple functional pathways of fungi. Biology (Basel) 12:513. https://doi.org/10.3390/biology12040513
Zhao H, Li J, Zhang Y et al (2018) Potential of iturins as functional agents: safe, probiotic, and cytotoxic to cancer cells. Food Funct 9:5580–5587. https://doi.org/10.1039/c8fo01523f
Aimaier R, Li H, Cao W et al (2023) The secondary metabolites of Bacillus subtilis strain Z15 induce apoptosis in hepatocellular carcinoma cells. Probiotics and Antimicrobial Proteins. https://doi.org/10.1007/s12602-023-10181-4
Blanchard OL, Smoliga JM (2015) Translating dosages from animal models to human clinical trials-revisiting body surface area scaling. Faseb J 29:1629–1634. https://doi.org/10.1096/fj.14-269043
Jiang Q, Hao R, Wang W et al (2016) SIRT1/Atg5/autophagy are involved in the antiatherosclerosis effects of ursolic acid. Mol Cell Biochem 420:171–184. https://doi.org/10.1007/s11010-016-2787-x
Ding S, Hong Y (2020) The fluorescence toolbox for visualizing autophagy. Chem Soc Rev 49:8354–8389. https://doi.org/10.1039/d0cs00913j
Olusola P, Banerjee HN, Philley JV, Dasgupta S (2019) Human papilloma virus-associated cervical cancer and health disparities. Cells 8:622. https://doi.org/10.3390/cells8060622
Gökalp F (2021) The effective natural compounds for inhibiting cervical cancer. Med Oncol 38:12. https://doi.org/10.1007/s12032-021-01456-3
Tank JG, Pandya RV (2022) Anti-proliferative activity of surfactins on human cancer cells and their potential use in therapeutics. Peptides 155:170836. https://doi.org/10.1016/j.peptides.2022.170836
Vo TTT, Liu J-F, Wu C-Z et al (2020) Surfactin from Bacillus subtilis induces apoptosis in human oral squamous cell carcinoma through ROS-regulated mitochondrial pathway. J Cancer 11:7253–7263. https://doi.org/10.7150/jca.50835
Li S, Ma Y-M, Zheng P-S, Zhang P (2018) GDF15 promotes the proliferation of cervical cancer cells by phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp Clin Cancer Res 37:80. https://doi.org/10.1186/s13046-018-0744-0
Tamura K (2015) Development of cell-cycle checkpoint therapy for solid tumors. Jpn J Clin Oncol 45:1097–1102. https://doi.org/10.1093/jjco/hyv131
Icard P, Fournel L, Wu Z et al (2019) Interconnection between metabolism and cell cycle in cancer. Trends Biochem Sci 44:490–501. https://doi.org/10.1016/j.tibs.2018.12.007
Suski JM, Braun M, Strmiska V, Sicinski P (2021) Targeting cell-cycle machinery in cancer. Cancer Cell 39:759–778. https://doi.org/10.1016/j.ccell.2021.03.010
Engeland K (2022) Cell cycle regulation: p53–p21-RB signaling. Cell Death Differ 29:946–960. https://doi.org/10.1038/s41418-022-00988-z
Yoshida GJ (2017) Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: from pathophysiology to treatment. J Hematol Oncol 10:67. https://doi.org/10.1186/s13045-017-0436-9
Nowosad A, Jeannot P, Callot C et al (2021) Publisher Correction: p27 controls Ragulator and mTOR activity in amino acid-deprived cells to regulate the autophagy–lysosomal pathway and coordinate cell cycle and cell growth. Nat Cell Biol 23:1048–1048. https://doi.org/10.1038/s41556-021-00741-7
Andrade-Tomaz M, de Souza I, Rocha CRR, Gomes LR (2020) The role of chaperone-mediated autophagy in cell cycle control and its implications in cancer. Cells 9:2140. https://doi.org/10.3390/cells9092140
Liu S, Yao S, Yang H et al (2023) Autophagy: regulator of cell death. Cell Death Dis 14:648. https://doi.org/10.1038/s41419-023-06154-8
Peng F, Liao M, Qin R et al (2022) Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther 7:286. https://doi.org/10.1038/s41392-022-01110-y
Tong X, Tang R, Xiao M et al (2022) Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol 15:174. https://doi.org/10.1186/s13045-022-01392-3
Denton D, Kumar S (2019) Autophagy-dependent cell death. Cell Death Differ 26:605–616. https://doi.org/10.1038/s41418-018-0252-y
Garnica P, Encío I, Plano D et al (2019) Organoseleno cytostatic derivatives: autophagic cell death with AMPK and JNK activation. Eur J Med Chem 175:234–246. https://doi.org/10.1016/j.ejmech.2019.04.074
Deng S, Shanmugam MK, Kumar AP et al (2019) Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 125:1228–1246. https://doi.org/10.1002/cncr.31978
Liu J, Liu P, Xu T et al (2020) Berberine induces autophagic cell death in acute lymphoblastic leukemia by inactivating AKT/mTORC1 signaling. Drug Des Devel Ther 14:1813–1823. https://doi.org/10.2147/DDDT.S239247
Hsieh C-L, Huang H-S, Chen K-C et al (2020) A novel salicylanilide derivative induces autophagy cell death in castration-resistant prostate cancer via ER stress-activated PERK signaling pathway. Mol Cancer Ther 19:101–111. https://doi.org/10.1158/1535-7163.MCT-19-0387
Funding
Natural Science Foundation of China,No.32160074,Open project of Key Laboratory in Xinjiang,No.2020D4010
Author information
Authors and Affiliations
Contributions
Conceptualization, Huixin Zhao, Qian Zhou and Xiufeng Pang; Data curation, Heping Zhao, Reyihanguli Aimaier and JunYang; Formal analysis, Dongyuan Zhou, Qian Zhou and Huixin Zhao; Funding acquisition,Huixin Zhao; Investigation, Haoran Li and Weiquan Wan; Methodology,Jinyu Li, Haoran Li; Project administration, Huixin Zhao; Resources, Huixin Zhao; Supervision, Xiufeng Pang; Writing-original draft, Haoran Li and Dongyuan Zhou; Writing -review & editing, Huixin Zhao.Huixin Zhao is the first corresponding author, and Qian Zhou and Professor Xiufeng Pang are the co corresponding authors. Haoran Li, Dongyuan Zhou and Weiquan Wang these authors contributed equally to this work and share first authorship. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Zhou, D., Wang, W. et al. Mycosubtilin Induces G1 Phase Block and Autophagy in Cervical Cancer HeLa Cells. Probiotics & Antimicro. Prot. (2025). https://doi.org/10.1007/s12602-025-10534-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-025-10534-1