Abstract
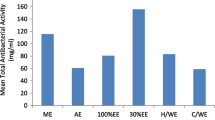
Kratom (Mitragyna speciosa) leaves are commonly used to enhance endurance and treat various diseases. This study evaluated the effect of kratom leaf fermentation with Lactobacillus rhamnosus. Antibacterial activity was investigated against Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Escherichia coli, and E. coli O157:H7. Biofilm inhibition and eradication assays were also performed. Antioxidant properties were determined by measuring the total phenolic and flavonoid content and DPPH and ABTS scavenging activities. Nitric oxide and TNF-α, IL-1β, and IL-6 expressions in LPS-stimulated RAW 264.7 macrophage cells were also measured. Aqueous kratom extract exhibited promising effects against free radicals and pro-inflammatory cytokines. Notably, all fermented kratoms showed significant antibacterial activity against the tested pathogens and antibiofilm formation by S. aureus and MRSA. Furthermore, the eradication of established biofilms of fermented kratoms was observed in S. aureus (day 2, 50 mg/mL) and E. coli (day 2, 100 mg/mL and day 4, 50 mg/mL). To the best of our knowledge, this study is the first to report that fermented and non-fermented kratoms could be nutraceutical sources of antibacterial, antibiofilm, antioxidant, and anti-inflammatory substances against related diseases and can be applied further in dietary or cosmetic products with health-promoting effects.




Similar content being viewed by others
Data Availability
This is not applicable.
References
Raffa RB (2014) Kratom and Other Mitragynines: The Chemistry and Pharmacology of Opioids from a Non-opium Source; CRC Press
Suwanlert S (1975) A study of kratom eaters in Thailand. Bull Narc 27(3):21–27
Charoenratana S, Anukul C, Aramrattana A (2021) Attitudes towards kratom use, decriminalization and the development of a community-based kratom control mechanism in Southern Thailand. Int J Drug Policy 95:103197
Cinosi E, Martinotti G, Simonato P, Singh D, Demetrovics Z, Roman-Urrestarazu A et al (2015) Following “the roots” of kratom (Mitragyna speciosa): the evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in Western countries. Biomed Res Int 2015:968786
Thanat N, Monthaka T, Siriporn P, Kwanroen S, Nootim P (2016) Traditional use of kratom (Mitragyna speciosa Korth) among folk healers in Southern Thailand. J Thai Tradition Altern Med 14(3):274–84
Tanguay P (2011) Kratom in Thailand. Available from: https://ssrn.com/abstract=1908849 or https://doi.org/10.2139/ssrn.1908849
Panyaphu D, Namkeard S, Inchai W, Kratom YK (2016) herbal medicine or nacrotic drug. Journal of Thai Traditional & Alternative Medicine 14(3):242–256
Meireles V, Rosado T, Barroso M, Soares S, Gonçalves J, Luís Â, Caramelo D, Simão AY, Fernández N, Duarte AP, Gallardo E (2019) Mitragyna speciosa: Clinical, toxicological aspects and analysis in biological and non-biological samples. Medicines (Basel, Switzerland) 6(1):35
Todd DA, Kellogg JJ, Wallace ED, Khin M, Flores-Bocanegra L, Tanna RS et al (2020) Chemical composition and biological effects of kratom (Mitragyna speciosa): in vitro studies with implications for efficacy and drug interactions. Sci Rep 10(1):19158
Prevete E, Kuypers KPC, Theunissen EL, Corazza O, Bersani G, Ramaekers JG (2022) A systematic review of (pre)clinical studies on the therapeutic potential and safety profile of kratom in humans. Hum Psychopharmacol Clin Exp 37(1):e2805
Adebo OA, Gabriela Medina-Meza I (2020) Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules (Basel, Switzerland) 25(4):927
Ranadheera C, Vidanarachchi J, Rocha R, Cruz A, Ajlouni S (2017) Probiotic delivery through fermentation: Dairy vs. non-dairy beverages. Fermentation 3(4):67
Patil A, Munot N, Patwekar M, Patwekar F, Ahmad I, Alraey Y et al (2022) Encapsulation of lactic acid bacteria by lyophilisation with its effects on viability and adhesion properties. Evid Based Complement Alternat Med 2022:4651194
Septembre-Malaterre A, Remize F, Poucheret P (2018) Fruits and vegetables, as a source of nutritional compounds and phytochemicals: changes in bioactive compounds during lactic fermentation. Food Res Int 104:86–99
Leonard W, Zhang P, Ying D, Adhikari B, Fang Z (2021) Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol Adv 49:107763
Kwon H-J, Ahn H, Kim BS, Kang S-S, Lee K-G (2022) Anti-bacterial and anti-inflammatory activities of lactic acid bacteria-bioconversioned indica rice (Oryza sativa L.) extract. Chem Biol Technol Agric 9(1):44
Jiang M, Deng K, Jiang C, Fu M, Guo C, Wang X et al (2016) Evaluation of the antioxidative, antibacterial, and anti-inflammatory effects of the aloe fermentation supernatant containing Lactobacillus plantarum HM218749.1. Mediators Inflamm 2016:2945650
López de Lacey AM, Pérez-Santín E, López-Caballero ME, Montero P (2014) Survival and metabolic activity of probiotic bacteria in green tea. LWT Food Sci Technol 55(1):314–22
Antolak H, Piechota D, Kucharska A (2021) Kombucha tea-a double power of bioactive compounds from tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 10(10):1541
Nissen L, di Carlo E, Gianotti A (2020) Prebiotic potential of hemp blended drinks fermented by probiotics. Food Res Int 131:109029
Nissen L, Casciano F, Babini E, Gianotti A (2021) Prebiotic potential and bioactive volatiles of hemp byproduct fermented by lactobacilli. LWT 151:112201
Romyasamit C, Saengsuwan P, Boonserm P, Thamjarongwong B, Singkhamanan K (2021) Optimization of cryoprotectants for freeze-dried potential probiotic Enterococcus faecalis and evaluation of its storage stability. Dry Technol 1–10
(CLSI) CaLSI (2022) CLSI supplement M100. In: James S. Lewis II P, FIDSA, editor. Performance Standards for Antimicrobial Susceptibility Testing. 32nd ed ed. USA
Davis JL (2018) Chapter 2 - pharmacologic principles. In: Reed SM, Bayly WM, Sellon DC, editors. Equine Internal Medicine (Fourth Edition): W.B. Saunders p 79–137
Sornsenee P, Chatatikun M, Mitsuwan W, Kongpol K, Kooltheat N, Sohbenalee S et al (2021) Lyophilized cell-free supernatants of Lactobacillus isolates exhibited antibiofilm, antioxidant, and reduces nitric oxide activity in lipopolysaccharide-stimulated RAW 264.7 cells. PeerJ 9:e12586
Chatatikun M, Supjaroen P, Promlat P, Chantarangkul C, Waranuntakul S, Nawarat J, ... Chiabchalard A (2020) Antioxidant and tyrosinase inhibitory properties of an aqueous extract of Garcinia atroviridis Griff. ex. T. Anderson fruit pericarps. Pharmacogn J 12:71–79
Asmerom D, Hailu GS, Yimer EM, Bitew H, Kahsay G (2020) Antimicrobial evaluation of latex and TLC fractions from the leaves of Aloe adigratana Reynolds. Evid Based Complement Alternat Med 2020:8312471
Vestby LK, Grønseth T, Simm R, Nesse LL (2020) Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 9(2):59
Crouzet M, Le Senechal C, Brözel VS, Costaglioli P, Barthe C, Bonneu M et al (2014) Exploring early steps in biofilm formation: set-up of an experimental system for molecular studies. BMC Microbiol 14(1):253
Landini P, Antoniani D, Burgess JG, Nijland R (2010) Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl Microbiol Biotechnol 86(3):813–823
Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9(1):522–554
Nadar S, Khan T, Patching SG, Omri A (2022) Development of antibiofilm therapeutics strategies to overcome antimicrobial drug resistance. Microorganisms 10(2):303
Schulze A, Mitterer F, Pombo JP, Schild S (2021) Biofilms by bacterial human pathogens: clinical relevance - development, composition and regulation - therapeutical strategies. Microb Cell 8(2):28–56
Ruhal R, Kataria R (2021) Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol Res 251:126829
Zhou L, Zhang Y, Ge Y, Zhu X, Pan J (2020) Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front Microbiol 11:589640
Seremet OC, Olaru OT, Gutu CM, Nitulescu GM, Ilie M, Negres S et al (2018) Toxicity of plant extracts containing pyrrolizidine alkaloids using alternative invertebrate models. Mol Med Rep 17(6):7757–7763
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C et al (2019) Chronic inflammation in the etiology of disease across the life span. Nat Med 25(12):1822–1832
Tripathi P, Tripathi P, Kashyap L, Singh V (2007) The role of nitric oxide in inflammatory reactions. FEMS Immunol Med Microbiol 51(3):443–452
Nunes CDR, Barreto Arantes M, Menezes de Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de Moraes L, Vieira IJC, Barros de Oliveira D (2020) Plants as sources of anti-inflammatory agents. Molecules 25(16):3726
Mocellin S, Bronte V, Nitti D (2007) Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med Res Rev 27(3):317–352
Yoshioka Y, Yamamuro A, Maeda S (2003) Nitric oxide at a low concentration protects murine macrophage RAW264 cells against nitric oxide-induced death via cGMP signaling pathway. Br J Pharmacol 139(1):28–34
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023
Maroon JC, Bost JW, Maroon A (2010) Natural anti-inflammatory agents for pain relief. Surg Neurol Int 1:80
Mo YN, Cheng F, Yang Z, Shang XF, Liang JP, Shang RF, Hao BC, Wang XH, Zhang HJ, Wali A, Lu CF, Liu Y (2021) Antioxidant activity and the potential mechanism of the fruit from Ailanthus altissima Swingle. Front Vet Sci 8:784898
Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, Zhang JJ, Li HB (2017) Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int J Mol Sci 18(1):96
Goh YS, Karunakaran T, Murugaiyah V, Santhanam R, Abu Bakar MH, Ramanathan S (2021) Accelerated Solvent Extractions (ASE) of Mitragyna speciosa Korth. (Kratom) leaves: Evaluation of its cytotoxicity and antinociceptive activity. Molecules 26(12):3704
Vuong QV, Hirun S, Roach PD, Bowyer MC, Phillips PA, Scarlett CJ (2013) Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J Herb Med 3(3):104–111
Avello MA, Pastene ER, Bustos ED, Bittner ML, Becerra JA (2013) Variation in phenolic compounds of Ugni molinae populations and their potential use as antioxidant supplement. Rev Bras 23(1):44–50
Goldsmith CD, Vuong QV, Stathopoulos CE, Roach PD, Scarlett CJ (2014) Optimization of the aqueous extraction of phenolic compounds from olive leaves. Antioxidants (Basel) 3(4):700–712
Bezerra ANS, Massing LT, de Oliveira RB, Mourão RHV (2017) Standardization and anti-inflammatory activity of aqueous extract of Psittacanthus plagiophyllus Eichl. (Loranthaceae). J Ethnopharmacol 202:234–40
Al-Manhel A, Niamah A (2015) Effect of aqueous and alcoholic plant extracts on inhibition of some types of microbes and causing spoilage of food. Pak J Food Sci 25:104–109
Patwekar FI, Patwekar M, Asif M, Heroor S, Mohsin AA (2010) Activity guided separation of phytoconstituents from the flowers of Ichnocarpus frutescens L. and evaluation for antioxidant property. 1:318–23
Manzur AGB, Sm Junior V, Morais-Costa F, Mariano EGA, Careli RT, da Silva LMV et al (2019) Extract of Mangifera indica L. leaves may reduce biofilms of Staphylococcus spp. in stainless steel and teatcup rubbers. Food Sci Technol Int 26(1):11–20
Parthasarathy S, Bin Azizi J, Ramanathan S, Ismail S, Sasidharan S, Said MI et al (2009) Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna speciosa (Rubiaceae family) leaves. Molecules 14(10):3964–3974
Chojnacka K, Lewandowska U (2022) Inhibition of pro-inflammatory cytokine secretion by polyphenol-rich extracts in macrophages via NF-κB pathway. Food Rev Int 1–20
Le Rouzic M, Bruniaux P, Raveschot C, Krier F, Phalip V, Ravallec R, … Coutte F (2023) Lactobacillus use for plant fermentation: New ways for plant-based product valorization. IntechOpen
Gustaw K, Niedźwiedź I, Rachwał K, Polak-Berecka M (2021) New Insight into bacterial interaction with the matrix of plant-based fermented foods. Foods 10(7):1603
Acknowledgements
We are grateful for the excellent support during the experiments from the Research Institute for Health Sciences Walailak University, School of Allied Health Sciences, Walailak University. We are extremely grateful to Dr. Jirakrit Saetang for their kind help and recommendation during the current study.
Funding
This study was supported by the Walailak University, funded by Dr. CBD Co., Ltd., grant number WUSTP-10/2565.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.R. and P.S.; methodology, C.R. and P.S.; software, C.R.; validation, C.R.; formal analysis, C.R. and S.C.; investigation, C.R.; resources, C.R.; data curation, C.R. and P.S.; writing—original draft preparation, C.R., S.C. and P.S.; writing—review and editing, C.R., S.C. and P.S.; visualization, C.R.; supervision, C.R.; project administration, C.R.; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sornsenee, P., Chimplee, S. & Romyasamit, C. Evaluation of Antibacterial, Antibiofilm, Antioxidant, and Anti-Inflammatory Activities of Kratom Leaves (Mitragyna speciosa) Fermentation Supernatant Containing Lactobacillus rhamnosus GG. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10142-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10142-x