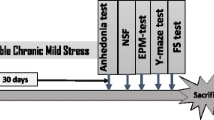
Abstract
Kefir is a probiotic mixture with anxiolytic and antioxidant properties. Chronic stress can lead to anxiety disorders and increase oxidative damage in organs such as the heart and kidney. In this study, we examined whether kefir ameliorates the anxiety-like behavior of mice submitted to chronic unpredictable stress (CUS) by modulating brain-derived neurotrophic factor (BDNF) and corticosterone levels and whether kefir modifies the oxidative parameters in the heart and kidney of mice. Male Swiss mice received kefir (0.3 mL/100 g/day) or milk for 30 days (gavage). On the 10th day, the mice were submitted to CUS. Behavioral analysis was performed using the elevated plus maze and forced swimming tests. BDNF levels were analyzed in brain tissues. Heart and kidney superoxide dismutase (SOD), catalase, glutathione (GSH), thiobarbituric acid reactive substances (TBARS), 3-nitrotyrosine, metalloproteinase-2 (MMP-2), and plasma corticosterone were evaluated. Kefir reverted the CUS-induced decrease in the time spent in the open arms, the increase in grooming frequency, and decrease in the head dipping frequency, but not the reduced immobility time. CUS decreased the cerebellum BDNF levels and increased corticosterone levels, which were restored by Kefir. Neither catalase and SOD activities nor GSH, TBARS, 3-nitrotyrosine, and MMP-2 were modified by CUS in the heart. In the kidney, CUS increased 3-nitrotyrosine and MMP-2. Kefir increased the antioxidant defense in the heart and kidney of control and CUS mice. These results suggest that kefir ameliorated CUS-induced anxiety-like behavior by modulating brain BDNF and corticosterone levels. Kefir also increased the antioxidant defense of mice heart and kidney.





Similar content being viewed by others
Data Availability
All data will be made available upon reasonable academic request within the limitations of informed consent by the corresponding author upon acceptance.
References
Lähdepuro A et al (2019) The impact of early life stress on anxiety symptoms in late adulthood. Sci Rep 9(1):1–13. https://doi.org/10.1038/s41598-019-40698-0
Yang CR et al (2020) Antidepressant drugs correct the imbalance between proBDNF/p75NTR/sortilin and mature BDNF/TrkB in the brain of mice with chronic stress. Neurotox Res 37(1):171–182. https://doi.org/10.1007/s12640-019-00101-2
Yu H, Tang M, Zeng Z, Huang S, Zheng X, Liu Z (2022) Suppressive effects of gelsemine on anxiety-like behaviors induced by chronic unpredictable mild stress in mice. Brain Sci. 12(2). https://doi.org/10.3390/brainsci12020191
Camuso S, La Rosa P, Fiorenza MT, Canterini S (2022) Pleiotropic effects of BDNF on the cerebellum and hippocampus: implications for neurodevelopmental disorders. Neurobiol Dis 163:105606. https://doi.org/10.1016/j.nbd.2021.105606
Alzoubi KH, Abdel-Hafiz L, Khabour OF, El-Elimat T, Alzubi MA, Alali FQ (2020) Evaluation of the effect of hypericum triquetrifolium turra on memory impairment induced by chronic psychosocial stress in rats: role of BDNF. Drug Des Devel Ther 14:5299–5314. https://doi.org/10.2147/DDDT.S278153
Marin MT, Cruz FC, Planeta CS (2007) Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav 90(1):29–35. https://doi.org/10.1016/j.physbeh.2006.08.021
Parul et al (2021) Chronic unpredictable stress negatively regulates hippocampal neurogenesis and promote anxious depression-like behavior via upregulating apoptosis and inflammatory signals in adult rats. Brain Res Bull 172(April):164–179. https://doi.org/10.1016/j.brainresbull.2021.04.017
Herman JP et al (2016) Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology. Compr Physiol 6(2):603–621. https://doi.org/10.1002/cphy.c150015.Regulation
Mehta AJ (2016) Alcoholism and critical illness: a review. World J Crit Care Med 5(1):27. https://doi.org/10.5492/wjccm.v5.i1.27
Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F (2017) Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 87:223–229. https://doi.org/10.1016/j.biopha.2016.12.105
Şahin E, Gümüşlü S (2007) Immobilization stress in rat tissues: alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comp Biochem Physiol - C Toxicol Pharmacol 144(4):342–347. https://doi.org/10.1016/j.cbpc.2006.10.009
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Rosa DD, Dias MMS, Grześkowiak ŁM, Reis SA, Conceição LL, Peluzio MDCG (2017) Milk kefir: nutritional, microbiological and health benefits. Nutr Res Rev 30(1):82–96. https://doi.org/10.1017/S0954422416000275
Mörkl S et al (2020) The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology 79(1):80–88. https://doi.org/10.1159/000492834
Noori N, Bangash M, Motaghinejad M, Hosseini P, Noudoost B (2014) Kefir protective effects against nicotine cessation-induced anxiety and cognition impairments in rats. Adv Biomed Res 3(1):251. https://doi.org/10.4103/2277-9175.146377
Chen H-L et al (2021) Kefir peptides exhibit antidepressant-like activity in mice through the BDNF/TrkB pathway. J Dairy Sci 104(6):6415–6430. https://doi.org/10.3168/jds.2020-19222
Pimenta FS et al (2018) Mechanisms of action of kefir in chronic cardiovascular and metabolic diseases. Cell Physiol Biochem 48(5):1901–1914. https://doi.org/10.1159/000492511
Punaro GR et al (2014) Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide - Biol Chem 37(1):53–60. https://doi.org/10.1016/j.niox.2013.12.012
Fahmy HA, Ismail AFM (2015) Gastroprotective effect of kefir on ulcer induced in irradiated rats. J Photochem Photobiol B Biol 144:85–93. https://doi.org/10.1016/j.jphotobiol.2015.02.009
Barboza KRM et al (2018) Gastroprotective effect of oral kefir on indomethacin-induced acute gastric lesions in mice: impact on oxidative stress. Life Sci 209(May):370–376. https://doi.org/10.1016/j.lfs.2018.08.035
Chen YH et al (2020) Anti-inflammatory, antioxidant, and antifibrotic effects of kefir peptides on salt-induced renal vascular damage and dysfunction in aged stroke-prone spontaneously hypertensive rats. Antioxidants 9(9):1–18. https://doi.org/10.3390/antiox9090790
Erdogan FS, Ozarslan S, Guzel-Seydim ZB, Kök Taş T (2019) The effect of kefir produced from natural kefir grains on the intestinal microbial populations and antioxidant capacities of Balb/c mice. Food Res Int 115(June 2018):408–413. https://doi.org/10.1016/j.foodres.2018.10.080
Strada R et al (2010) Microbial profile of a kefir sample preparations – grains in natura and lyophilized and fermented suspension. Ciência e Tecnol Aliment 30(4):1022–1026
Brasil GA et al (2018) The benefits of soluble non-bacterial fraction of kefir on blood pressure and cardiac hypertrophy in hypertensive rats are mediated by an increase in baroreflex sensitivity and decrease in angiotensin-converting enzyme activity. Nutrition 51–52:66–72. https://doi.org/10.1016/j.nut.2017.12.007
Lepsch LB, Gonzalo LA, Magro FJB, Delucia R, Scavone C, Planeta CS (2005) Exposure to chronic stress increases the locomotor response to cocaine and the basal levels of corticosterone in adolescent rats. Addict Biol 10(3):251–256. https://doi.org/10.1080/13556210500269366
Cruz FC, Marin MT, Leão RM, Planeta CS (2012) Behavioral and neuroendocrine effects of the exposure to chronic restraint or variable stress in early adolescent rats. Int J Dev Neurosci 30(1):19–23. https://doi.org/10.1016/j.ijdevneu.2011.10.005
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14(3):149–167. https://doi.org/10.1016/0165-0270(85)90031-7
Almeida CAF et al (2021) Ayahuasca, a psychedelic beverage, modulates neuroplasticity induced by ethanol in mice. Behav Brain Res 416(August):2022. https://doi.org/10.1016/j.bbr.2021.113546
Dalvi A, Rodgers RJ (1999) Behavioral effects of diazepam in the murine plus-maze: flumazenil antagonism of enhanced head dipping but not the disinhibition of open-arm avoidance. Pharmacol Biochem Behav 62(4):727–734. https://doi.org/10.1016/S0091-3057(98)00220-2
Arakawa H (2021) Implication of the social function of excessive self-grooming behavior in BTBR T+ltpr3tf/J mice as an idiopathic model of autism. Physiol Behav 237:113432. https://doi.org/10.1016/j.physbeh.2021.113432
Reimer AE, de Oliveira AR, Diniz JB, Hoexter MQ, Chiavegatto S, Brandão ML (2015) Rats with differential self-grooming expression in the elevated plus-maze do not differ in anxiety-related behaviors. Behav Brain Res 292:370–380. https://doi.org/10.1016/j.bbr.2015.06.036
Kliethermes CL, Crabbe JC (2006) Pharmacological and genetic influences on hole-board behaviors in mice. Pharmacol Biochem Behav 85(1):57–65. https://doi.org/10.1016/j.pbb.2006.07.007
Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC (2016) Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 17(1):45–59. https://doi.org/10.1038/nrn.2015.8
Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD (2011) The mouse forced swim test. J Vis Exp 58:4–8. https://doi.org/10.3791/3638
Do Valle GT, Ricci ST, Silva AO, Tirapelli CR, Ceron CS (2020) Ethanol consumption increases renal dysfunction and mortality in a mice model of sub-lethal sepsis. Can J Physiol Pharmacol pp. 1–35. https://doi.org/10.1139/cjpp-2020-0564.
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Simplicio JA, Resstel LB, Tirapelli DPC, D’Orléans-Juste P, Tirapelli CR (2015) Contribution of oxidative stress and prostanoids in endothelial dysfunction induced by chronic fluoxetine treatment. Vascul Pharmacol 73:124–137. https://doi.org/10.1016/j.vph.2015.06.015
Hu C et al (2017) Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS ONE 12(9):1–15. https://doi.org/10.1371/journal.pone.0185129
Mehta V, Parashar A, Udayabanu M (2017) Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol Behav 171:69–78. https://doi.org/10.1016/j.physbeh.2017.01.006
López-López AL, Jaime HB, Villanueva MD, Padilla MB, Palacios GV, Aguilar FJ (2016) Chronic unpredictable mild stress generates oxidative stress and systemic inflammation in rats. Physiol Behav 161(186):15–23. https://doi.org/10.1016/j.physbeh.2016.03.017
Liu X et al (2021) Impact of inosine on chronic unpredictable mild stress-induced depressive and anxiety-like behaviors with the alteration of gut microbiota. Front Cell Infect Microbiol 11(September):1–11. https://doi.org/10.3389/fcimb.2021.697640
Marangon PB, Silva LE, Rorato R, Gomiero Alves P, Antunes‐Rodrigues J, Elias LL (2014) Oestradiol modulates the effects of leptin on energy homeostasis by corticotrophin-releasing factor type 2 receptor. J Neuroendocrinol 26(11):796–804. https://doi.org/10.1111/jne.12192
da Silva Marques JG et al (2021) Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav Brain Res 399. https://doi.org/10.1016/j.bbr.2020.113002.
Li H, Wang P, Huang L, Li P, Zhang D (2019) Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol Motil 31(10):1–13. https://doi.org/10.1111/nmo.13677
Schmidt HD, Duman RS (2010) Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 35(12):2378–2391. https://doi.org/10.1038/npp.2010.114
Govindarajan A et al (2006) Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A 103(35):13208–13213. https://doi.org/10.1073/pnas.0605180103
Lin L, Herselman MF, Zhou XF, Bobrovskaya L (2022) Effects of corticosterone on BDNF expression and mood behaviours in mice. Physiol Behav vol. 247(November 2021):113721. https://doi.org/10.1016/j.physbeh.2022.113721
Liu X, Cao S, Zhang X (2015) Modulation of gut microbiota-brain axis by probiotics, prebiotics, and diet. J Agric Food Chem 63(36):7885–7895. https://doi.org/10.1021/acs.jafc.5b02404
Li Y, Peng C, Guo X, You JJ, Yadav HP (2015) Expression of brain-derived neurotrophic factor and tyrosine kinase B in cerebellum of poststroke depression rat model. Chin Med J (Engl) 128(21):2926–2931. https://doi.org/10.4103/0366-6999.168058
Ishola IO, Olubodun-Obadun TG, Bakre OA, Ojo ES, Adeyemi OO (2022) Kolaviron ameliorates chronic unpredictable mild stress-induced anxiety and depression: involvement of the HPA axis, antioxidant defense system, cholinergic, and BDNF signaling. Drug Metab Pers Ther. https://doi.org/10.1515/dmpt-2021-0125
Li J, He P, Zhang J, Li N (2021) Orcinol glucoside improves the depressive-like behaviors of perimenopausal depression mice through modulating activity of hypothalamic–pituitary–adrenal/ovary axis and activating BDNF-TrkB-CREB signaling pathway. Phyther Res 35(10):5795–5807. https://doi.org/10.1002/ptr.7237
Thakare VN, Patil RR, Oswal RJ, Dhakane VD, Aswar MK, Patel BM (2018) Therapeutic potential of silymarin in chronic unpredictable mild stress induced depressive-like behavior in mice. J Psychopharmacol 32(2):223–235. https://doi.org/10.1177/0269881117742666
Wang D et al (2022) Tryptophan-rich diet ameliorates chronic unpredictable mild stress induced depression- and anxiety-like behavior in mice: the potential involvement of gut-brain axis. Food Res Int 157:111289. https://doi.org/10.1016/j.foodres.2022.111289
Luna RA, Foster JA (2015) Gut brain axis: diet microbiota interactions and implications for modulation of anxiety and depression. Curr Opin Biotechnol 32(Figure 1):35–41. https://doi.org/10.1016/j.copbio.2014.10.007
O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF (2015) Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277:32–48. https://doi.org/10.1016/j.bbr.2014.07.027
Marrocco J et al (2017) A sexually dimorphic pre-stressed translational signature in CA3 pyramidal neurons of BDNF Val66Met mice. Nat Commun 8(1):1–15. https://doi.org/10.1038/s41467-017-01014-4
Furman O, Tsoory M, Chen A (2022) Differential chronic social stress models in male and female mice. Eur J Neurosci 55(9–10):2777–2793. https://doi.org/10.1111/ejn.15481
Sánchez-Solís CN, Cuevas-Romero E, Munoz A, Cervantes-Rodríguez M, Rodríguez-Antolín J, Nicolás-Toledo L (2018) Morphometric changes and AQP2 expression in kidneys of young male rats exposed to chronic stress and a high-sucrose diet. Biomed Pharmacother 105(June):1098–1105. https://doi.org/10.1016/j.biopha.2018.06.086
Zhu LJ, Zhang CC, Chen C (2021) Research progress on the vesicle cycle and neurological disorders. J Pharm Pharm Sci 24(9):400–412. https://doi.org/10.18433/jpps31458
Yener AU et al (2015) Effects of kefir on ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci 19(5):887–896
Mert H, Yilmaz H, Irak K, Yildirim S, Mert N (2018) Investigation of the protective effect of kefir against isoproterenol induced myocardial infarction in rats. Korean J Food Sci Anim Resour 38(2):259–272. https://doi.org/10.5851/kosfa.2018.38.2.259
Kandasamy AD, Chow AK, Ali MAM, Schulz R (2010) Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res 85(3):413–423. https://doi.org/10.1093/cvr/cvp268
Cavdar Z et al (2017) Protective effects of taurine against renal ischemia/reperfusion injury in rats by inhibition of gelatinases, MMP-2 and MMP-9, and p38 mitogen-activated protein kinase signaling. Biotech Histochem 92(7):524–535. https://doi.org/10.1080/10520295.2017.1367033
Hu JB et al (2018) An E-selectin targeting and MMP-2-responsive dextran-curcumin polymeric prodrug for targeted therapy of acute kidney injury. Biomater Sci 6(12):3397–3409. https://doi.org/10.1039/c8bm00813b
Kim H et al (2019) Plant-based diets and incident CKD and kidney function. Clin J Am Soc Nephrol 14(5):682–691. https://doi.org/10.2215/CJN.12391018
Ferraro PM, Bargagli M, Trinchieri A, Gambaro G, Assimos DG (2020) Re: risk of kidney stones: influence of dietary factors, dietary patterns, and vegetarian-vegan diets. J Urol 204(3):612. https://doi.org/10.1097/JU.0000000000001173.01
Valim A, Carpes LS, Nicoletto BB (2022) Effect of vegetarian diets on renal function in patients with chronic kidney disease under non-dialysis treatment: a scoping review. Brazilian J Nephrol 44(3). https://doi.org/10.1590/2175-8239-jbn-2021-0126
Acknowledgements
The authors acknowledge the support of Alexandre Giusti Paiva and Giovane Galdino.
Funding
The research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq, 406177/2016–3), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil (FAPEMIG, PPM-00383–18), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Contributions
Alessandra Oliveira Silva, Larissa Helena Torres, and Carla Speroni Ceron conceived and designed the research. Alessandra Oliveira Silva, Jéssyca Milene Ribeiro, Talita Barbará Patrocínio, Gabriel Estevam Amorim, Antônio Alves Pereira-Júnior, and Marilene Lopes Ângelo conducted experiments. Fernanda Borges de Araújo Paula, Marcos Vinícios Salles Dias, Nelma de Mello Silva Oliveira, Larissa Helena Torres, José Antunes-Rodrigues, and Lucila Leico Kagohara Elias contributed with reagents or analytical tools. Alessandra Oliveira Silva, Sílvia Graciela Ruginsk and Carla Speroni Ceron analyzed data. Alessandra Oliveira Silva and Carla Speroni Ceron wrote the manuscript. All authors reviewed and approved the manuscript. All authors of the paper have fulfilled the criteria for authorship.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, A.O., Ribeiro, J.M., Patrocínio, T.B. et al. Protective Effects of Kefir Against Unpredictable Chronic Stress Alterations in Mice Central Nervous System, Heart, and Kidney. Probiotics & Antimicro. Prot. 15, 411–423 (2023). https://doi.org/10.1007/s12602-022-10031-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-10031-9