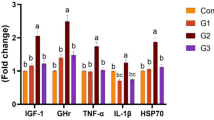
Abstract
Probiotics or direct-fed microbials (DFM) have proven strong potential for improving aquaculture sustainability. This study aims to evaluate the effects of dietary supplementation with the DFM Bacillus amyloliquefaciens US573 on growth performance, intestinal morphology, and gut microbiota (GM) of European sea bass. For this purpose, healthy fish were divided into two feeding trials in triplicate of 25 fish in each tank. The fish were fed with a control basal diet or a DFM-supplemented diet for 42 days. Results showed that, while no significant effects on growth performance were observed, the length and abundance of villi were higher in the DFM-fed group. The benefic effects of DFM supplementation included also the absence of cysts formation and the increase in number of goblet cells playing essential role in immune response. Through DNA metabarcoding analysis of GM, 5 phyla and 14 major genera were identified. At day 42, the main microbiome changes in response to B. amyloliquefaciens US573 addition included the significant decrease in abundance of Actinobacteria phylum that perfectly correlates with a decrease in Nocardia genus representatives which represent serious threat in marine and freshwater fish. On the contrary, an obvious dominance of Betaproteobacteria associated with the abundance in Variovorax genus members, known for their ability to metabolize numerous substrates, was recorded. Interestingly, Firmicutes, particularly species affiliated to the genus Sporosarcina with recent promising probiotic potential, were identified as the most abundant. These results suggest that B. amyloliquefaciens US573 can be effectively recommended as health-promoting DFM in European sea bass farming.






Similar content being viewed by others
Data Availability
All 16S rRNA Illumina amplicon sequencing data presented in this study are openly available in the Short Read Archive of the National Centre for Biotechnology Information (NCBI) with accession number PRJNA800410.
References
FAO (2019) FAO aquaculture newsletter. No. 60 (August). Rome
Watts JE, Schreier HJ, Lanska L et al (2017) The rising tide of antimicrobial resistance in aquaculture: sources, sinks and solutions. Mar Drugs 15(6):158. https://doi.org/10.3390/md15060158
Foysal MJ, Momtaz F, Robiul Kawser AQM et al (2019) Microbiome patterns reveal the transmission of pathogenic bacteria in hilsa fish (Tenualosa ilisha) marketed for human consumption in Bangladesh. J Appl Microbiol 126:1879–1890. https://doi.org/10.1111/jam.14257
Ng C, Gin KYH (2019) Monitoring antimicrobial resistance dissemination in aquatic systems. Water 11:1–7. https://doi.org/10.3390/w11010071
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture 446:88–96. https://doi.org/10.1016/j.aquaculture.2015.03.014
Choudhury TG, Kamilya D (2019) Paraprobiotics: an aquaculture perspective. Rev Aquac 11:1258–1270. https://doi.org/10.1111/raq.12290
Rimoldi S, Torrecillas S, Montero D et al (2020) Assessment of dietary supplementation with galactomannan oligosaccharides and phytogenics on gut microbiota of European sea bass (Dicentrarchus Labrax) fed low fishmeal and fish oil based diet. PLoS ONE 15:e0231494. https://doi.org/10.1371/journal.pone.0231494
El-Saadony MT, Alagawany M, Patra AK et al (2021) The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol 117:36–52. https://doi.org/10.1016/j.fsi.2021.07.007
FAO/WHO (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, a joint FAO/WHO consultation Cordoba, Argentina, 1–4 October
Hoseinifar SH, Sun YZ, Wang A, Zhou Z (2018) Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front Microbiol 9:1–18. https://doi.org/10.3389/fmicb.2018.02429
Chauhan A, Singh R (2019) Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis 77:99–113. https://doi.org/10.1007/s13199-018-0580-1
Tarnecki AM, Wafapoor M, Phillips RN, Rhody NR (2019) Benefits of a Bacillus probiotic to larval fish survival and transport stress resistance. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-39316-w
Foysal MJ, Alam M, Kawser AQMR et al (2020) Meta-omics technologies reveals beneficiary effects of Lactobacillus plantarum as dietary supplements on gut microbiota, immune response and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 520:734974. https://doi.org/10.1016/j.aquaculture.2020.734974
Van Doan H, Hoseinifar SH, Ringø E et al (2020) Host-associated probiotics: a key factor in sustainable aquaculture. Rev Fish Sci Aquacult 28:16–42. https://doi.org/10.1080/23308249.2019.1643288
Gobi N, Vaseeharan B, Chen JC et al (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol 74:501–508. https://doi.org/10.1016/j.fsi.2017.12.066
Ramesh D, Souissi S (2018) Effects of potential probiotic Bacillus subtilis KADR1 and its subcellular components on immune responses and disease resistance in Labeo rohita. Aquac Res 49:367–377. https://doi.org/10.1111/are.13467
Yi Y, Zhang Z, Zhao F et al (2018) Probiotic potential of Bacillus velezensis JW: antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish shellfish immunol 78:322–330. https://doi.org/10.1016/j.fsi.2018.04.055
Kuebutornye FK, Abarike ED, Lu Y (2019) A review on the application of Bacillus as probiotics in aquaculture. Fish shellfish immunol 87:820–828. https://doi.org/10.1016/j.fsi.2019.02.010
Kuebutornye FK, Abarike ED, Lu Y et al (2020) Mechanisms and the role of probiotic Bacillus in mitigating fish pathogens in aquaculture. Fish Physiol Biochem 46:819–841. https://doi.org/10.1007/s10695-019-00754-y
Wu Z, Qi X, Qu S, Ling F, Wang G (2021) Dietary supplementation of Bacillus velezensis B8 enhances immune response and resistance against Aeromonas veronii in grass carp. Fish Shellfish Immunol 115:14–21. https://doi.org/10.1016/j.fsi.2021.05.012
Goda AM, Omar EA, Srour TM et al (2018) Effect of diets supplemented with feed additives on growth, feed utilization, survival, body composition and intestinal bacterial load of early weaning European seabass, Dicentrarchus labrax post-larvae. Aquac Int 26:169–183. https://doi.org/10.1007/s10499-017-0200-8
Zokaeifar H, Babaei N, Saad CR et al (2014) Administration of Bacillus subtilis strains in the rearing water enhances the water quality, growth performance, immune response, and resistance against Vibrio harveyi infection in juvenile white shrimp, Litopenaeus vannamei. Fish shellfish Immunol 36:68–74. https://doi.org/10.1016/j.fsi.2013.10.007
Eissa N, Wang HP, Yao H, Abou-ElGheit E (2018) Mixed Bacillus species enhance the innate immune response and stress tolerance in yellow perch subjected to hypoxia and air-exposure stress. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-25269-z
Olmos J, Acosta M, Mendoza G, Pitones V (2020) Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch Microbiol 202:427–435. https://doi.org/10.1007/s00203-019-01757-2
Yánez-Mendizábal V, Viñas I, Usall J et al (2012) Formulation development of the biocontrol agent Bacillus subtilis strain CPA-8 by spray-drying. J Appl Microbiol 112:954–965. https://doi.org/10.1111/j.1365-2672.2012.05258.x
Diwan AD, Harke SN, Panche AN (2021) Aquaculture industry prospective from gut microbiome of fish and shellfish: an overview. J Anim Physiol Anim Nutr 00:1–29. https://doi.org/10.1111/jpn.13619
Giatsis C, Sipkema D, Ramiro-Garcia J, Bacanu GM et al (2016) Probiotic legacy effects on gut microbial assembly in tilapia larvae. Sci Rep 6:1–11. https://doi.org/10.1038/srep33965
Jiang Y, Wang Y, Zhang Z et al (2019) Responses of microbial community structure in turbot (Scophthalmus maximus) larval intestine to the regulation of probiotic introduced through live feed. PLoS ONE 14(5):e0216590. https://doi.org/10.1371/journal.pone.0216590
Maas RM, Deng Y, Dersjant-Li Y et al (2021) Exogenous enzymes and probiotics alter digestion kinetics, volatile fatty acid content and microbial interactions in the gut of Nile tilapia. Sci Rep 11:1–16. https://doi.org/10.1038/s41598-021-87408-3
Vandeputte M, Gagnaire PA, Allal F (2019) The European sea bass: a key marine fish model in the wild and in aquaculture. Anim Genet 50:195–206. https://doi.org/10.1111/age.12779
FAO (2018) Fisheries statistics and information. In: FAO Fisheries and Aquaculture Department. FAO, Rome: http://www.fao.org/fishery/statistics/en
Nikouli E, Meziti A, Antonopoulou E et al (2018) Gut bacterial communities in geographically distant populations of farmed sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax). Microorganisms 6:92. https://doi.org/10.3390/microorganisms6030092
Kokou F, Sasson G, Friedman J et al (2019) Core gut microbial communities are maintained by beneficial interactions and strain variability in fish. Nat Microbiol 4:2456–2465. https://doi.org/10.1038/s41564-019-0560-0
Kokou F, Sasson G, Mizrahi I, Cnaani A (2020) Antibiotic effect and microbiome persistence vary along the European seabass gut. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-66622-5
Serra CR, Oliva-Teles A, Enes P, Tavares F (2021) Gut microbiota dynamics in carnivorous European seabass (Dicentrarchus labrax) fed plant-based diets. Sci Rep 11:1–13. https://doi.org/10.1038/s41598-020-80138-y
Busti S, Rossi B, Volpe E et al (2020) Effects of dietary organic acids and nature identical compounds on growth, immune parameters and gut microbiota of European sea bass. Sci Rep 10(1):21321. https://doi.org/10.1038/s41598-020-78441-9
Pérez-Pascual D, Estellé J, Dutto G et al (2020) Growth performance and adaptability of European sea bass (Dicentrarchus labrax) gut microbiota to alternative diets free of fish products. Microorganisms 8(9):1346. https://doi.org/10.3390/microorganisms8091346
Farhat-Khemakhem A, Blibech M, Boukhris I et al (2018) Assessment of the potential of the multi-enzyme producer Bacillus amyloliquefaciens US573 as alternative feed additive. J Sci Food Agric 98:1208–1215. https://doi.org/10.1002/jsfa.8574
Guo X, Li D, Lu W et al (2006) Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Antonie Van Leeuwenhoek 90:139–146. https://doi.org/10.1007/s10482-006-9067-9
Posada-Uribe LF, Romero-Tabarez M, Villegas-Escobar V (2015) Effect of medium components and culture conditions in Bacillus subtilis EA-CB0575 spore production. Bioprocess Biosyst Eng 38:1879–1888. https://doi.org/10.1007/s00449-015-1428-1
Abdallah MB, Karray F, Mhiri N et al (2016) Prokaryotic diversity in a Tunisian hypersaline lake, Chott El Jerid. Extremophiles 20:125–138. https://doi.org/10.1007/s00792-015-0805-7
Marathe N, Shetty S, Lanjekar V et al (2012) Changes in human gut flora with age: an Indian familial study. BMC Microbiol 12:1–10. https://doi.org/10.1186/1471-2180-12-222
Takahashi S, Tomita J, Nishioka K et al (2014) Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE 9:e105592. https://doi.org/10.1371/journal.pone.0105592
Caporaso JG, Kuczynski J, Stombaugh J et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Edgar RC, Haas BJ, Clemente JC et al (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana, p 96
Simpson EH (1949) Measurement of diversity. Nature 163:688–688. https://doi.org/10.1038/163688a0
Lozupone CA, Knight R (2008) Species divergence and the measurement of microbial diversity. FEMS Microbiol Rev 32:557–578. https://doi.org/10.1111/j.1574-6976.2008.00111.x
Langfelder P, Horvath S (2012) Fast R functions for robust correlations and hierarchical clustering. J Stat Softw 46(11):i11. https://doi.org/10.18637/jss.v046.i11
Lahti L, Shetty S (2012) Microbiome-GitHub. http://microbiome.github.com/microbiome
Serra CR, Earl AM, Barbosa TM et al (2014) Sporulation during growth in a gut isolate of Bacillus subtilis. J Bacteriol 196:4184–4196. https://doi.org/10.1128/JB.01993-14
Givens CE, Ransom B, Bano N, Hollibaugh JT (2015) Comparison of the gut microbiomes of 12 bony fish and 3 shark species. Mar Ecol Prog Ser 518:209–223. https://doi.org/10.3354/meps11034
Tarnecki AM, Burgos FA, Ray CL, Arias CR (2017) Fish intestinal microbiome: diversity and symbiosis unravelled by metagenomics. J Appl Microbiol 123:2–17. https://doi.org/10.1111/jam.13415
Gatesoupe FJ, Huelvan C, Le Bayon N et al (2016) The highly variable microbiota associated to intestinal mucosa correlates with growth and hypoxia resistance of sea bass, Dicentrarchus labrax, submitted to different nutritional histories. BMC Microbiol 16(1):266. https://doi.org/10.1186/s12866-016-0885-2
Ushakova NA, Pravdin VG, Kravtsova LZ et al (2021) Complex bioactive supplements for aquaculture-evolutionary development of probiotic concepts. Probiotics Antimicrob Proteins 13(6):1696–1708. https://doi.org/10.1007/s12602-021-09835-y
Soto JO (2017) Bacillus probiotic enzymes: external auxiliary apparatus to avoid digestive deficiencies, water pollution, diseases, and economic problems in marine cultivated animals. Adv Food Nutr Res 80:15–35. https://doi.org/10.1016/bs.afnr.2016.11.001
Soto JO (2021) Feed intake improvement, gut microbiota modulation and pathogens control by using Bacillus species in shrimp aquaculture. World J Microbiol Biotechnol 37:1–7. https://doi.org/10.1007/s11274-020-02987-z
Hasan MT, Jang WJ, Kim H et al (2018) Synergistic effects of dietary Bacillus sp. SJ-10 plus β-glucooligosaccharides as a synbiotic on growth performance, innate immunity and streptococcosis resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 82:544–553. https://doi.org/10.1016/j.fsi.2018.09.002
Jang WJ, Hasan MT, Lee BJ et al (2020) Effect of dietary differences on changes of intestinal microbiota and immune-related gene expression in juvenile olive flounder (Paralichthys olivaceus). Aquaculture 527:735442. https://doi.org/10.1016/j.aquaculture.2020.735442
González-Félix ML, Gatlin DM III, Urquidez-Bejarano P et al (2018) Effects of commercial dietary prebiotic and probiotic supplements on growth, innate immune responses, and intestinal microbiota and histology of Totoaba macdonaldi. Aquaculture 491:239–251. https://doi.org/10.1016/j.aquaculture.2018.03.031
Reda R, Selim K (2015) Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morphology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquac Int 23:203–217. https://doi.org/10.1007/s10499-014-9809-z
Ramos MA, Batista S, Pires MA et al (2017) Dietary probiotic supplementation improves growth and the intestinal morphology of Nile tilapia. Animal 11:1259–1269. https://doi.org/10.1017/S1751731116002792
Messina JP, Brady OJ, Golding N et al (2019) The current and future global distribution and population at risk of dengue. Nat Microbiol 4:1508–1515. https://doi.org/10.1038/s41564-019-0476-8
Islam SM, Rohani MF, Shahjahan M (2021) Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquac Rep 21:100800. https://doi.org/10.1016/j.aqrep.2021.100800
Harris JM (1993) The presence, nature, and role of gut microflora in aquatic invertebrates: a synthesis. Microb Ecol 25:195–231. https://doi.org/10.1007/BF00171889
Austin B (2006) The bacterial microflora of fish, revised. Sci World J 6:931–945. https://doi.org/10.1100/tsw.2006.181
Suo Y, Li E, Li T et al (2017) Response of gut health and microbiota to sulfide exposure in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 63:87–96. https://doi.org/10.1016/j.fsi.2017.02.008
Yang X, Xu M, Huang G et al (2019) Effect of dietary L-tryptophan on the survival, immune response and gut microbiota of the Chinese mitten crab. Eriocheir sinensis Fish Shellfish Immunol 84:1007–1017. https://doi.org/10.1016/j.fsi.2018.10.076
Liu X, Cao Y, Ouyang S, Wu X (2020) Comparative analysis of gut microbiota diversity in endangered, economical, and common freshwater mussels using 16S rRNA gene sequencing. Ecol Evol 10:12015–12023. https://doi.org/10.1002/ece3.6796
Niu KM, Khosravi S, Kothari D et al (2019) Effects of dietary multi-strain probiotics supplementation in a low fishmeal diet on growth performance, nutrient utilization, proximate composition, immune parameters, and gut microbiota of juvenile olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 93:258–268. https://doi.org/10.1016/j.fsi.2019.07.056
Zhou W, Liu B, Li Y et al (2021) Dietary supplementation with Sporosarcina aquimarina MS4 enhances juvenile sea cucumber (Apostichopus japonicus) growth, immunity and disease resistance against Vibrio splendidus infection at low temperature. Aquac Nutr 27:918–926. https://doi.org/10.1111/anu.13236
Anbu P, Kang CH, Shin YJ, So JS (2016) Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 5:1–26. https://doi.org/10.1186/s40064-016-1869-2
Zhang X, Fu L, Deng B et al (2013) Bacillus subtilis SC02 supplementation causes alterations of the microbial diversity in grass carp water. World J Microbiol Biotechnol 29(9):1645–1653. https://doi.org/10.1007/s11274-013-1327-z
Liu H, Li J, Guo X et al (2018) Yeast culture dietary supplementation modulates gut microbiota, growth and biochemical parameters of grass carp. Microb Biotechnol 11:551–565. https://doi.org/10.1111/1751-7915.13261
Gutiérrez-Falcón AI, Ramos-Nuez AM, de Los MY et al (2021) Probiotic properties of Alcaligenes faecalis isolated from Argyrosomus regius in experimental peritonitis (rat model). Probiotics Antimicrob Proteins 13(5):1326–1337. https://doi.org/10.1007/s12602-021-09767-7
Annamalai N, Kumar A, Saravanakumar A et al (2011) Characterization of protease from Alcaligens faecalis and its antibacterial activity on fish pathogens. J Environ Biol 32:781–786
Tsertou MI, Smyrli M, Kokkari C et al (2018) The aetiology of systemic granulomatosis in meagre (Argyrosomus regius): the “Nocardia” hypothesis. Aquac Rep 12:5–11. https://doi.org/10.1016/j.aqrep.2018.08.002
Acknowledgements
We are grateful to Dr. Mohammed Y. Refai, head of the Department of Biological Sciences at the College of Science, University of Jeddah, for his continuous support.
Funding
This work was supported by the Distinguished Scientific Researches project N° UJ-20–030-DR University of Jeddah.
Author information
Authors and Affiliations
Contributions
Conceptualization: Hamadi Guerbej, Fatma Karray, and Hichem Chouayekh. Investigation: Insaf Boubaker, Manel Ben Abdallah, Ameny Farhat-Khemakhem, and Hichem Chouayekh. Data analysis: Najla Mhiri, Ameny Farhat-Khemakhem, and Fatma Karray. Writing original draft: Ameny Farhat-Khemakhem. Writing—reviewing and editing: Fatma Karray, Othman A. Alghamdi, Hamadi Guerbej, and Hichem Chouayekh.
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments described in the present study were conducted at the National Institute of Science and Technology of the Sea (INSTM Monastir, Tunisia) in accordance with the recommendations of Directive 2010/63/EU on the protection of animals used for scientific purposes.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chouayekh, H., Farhat-Khemakhem, A., Karray, F. et al. Effects of Dietary Supplementation with Bacillus amyloliquefaciens US573 on Intestinal Morphology and Gut Microbiota of European Sea Bass. Probiotics & Antimicro. Prot. 15, 30–43 (2023). https://doi.org/10.1007/s12602-022-09974-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09974-w